Publications
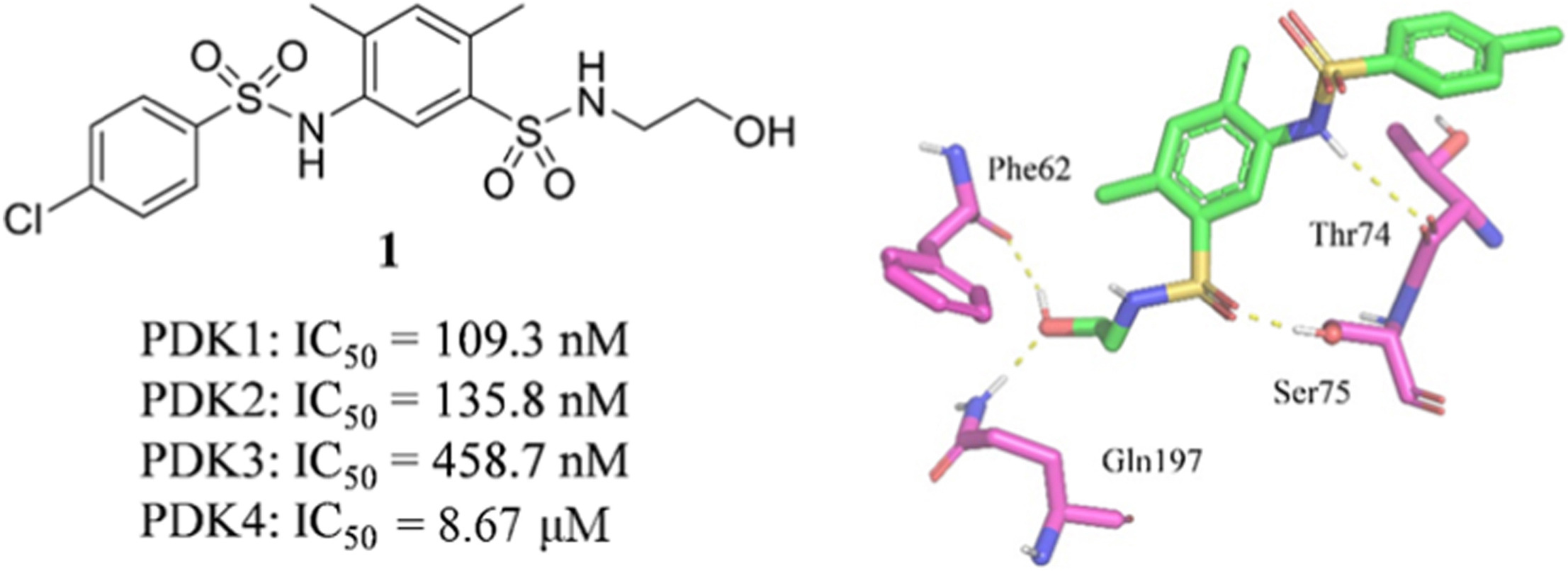
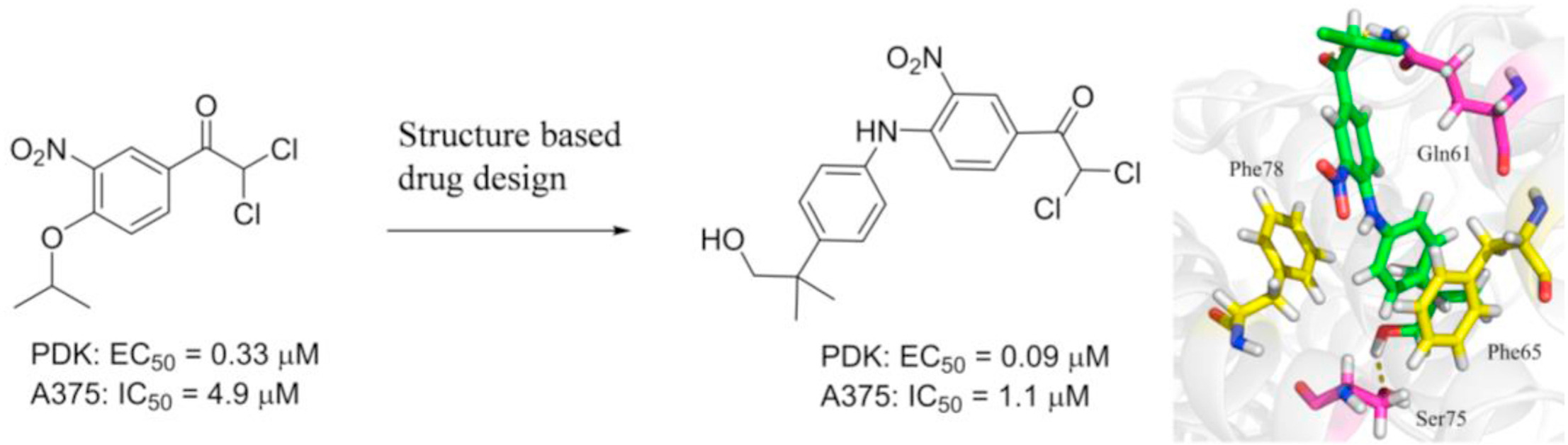
154. L. L. Gan, Y. Yang, Z. Z. Liang, M. J. Zhang, Y. He, S. L. Zhang, Targeting the pyruvate dehydrogenase complex/pyruvate dehydrogenase kinase (PDC/PDK) axis to discover potent PDK inhibitors through structure-based virtual screening and pharmacological evaluation, Eur. J. Med. Chem., 2023, 261, 116008. DOI:10.1016/j.ejmech.2023.116008
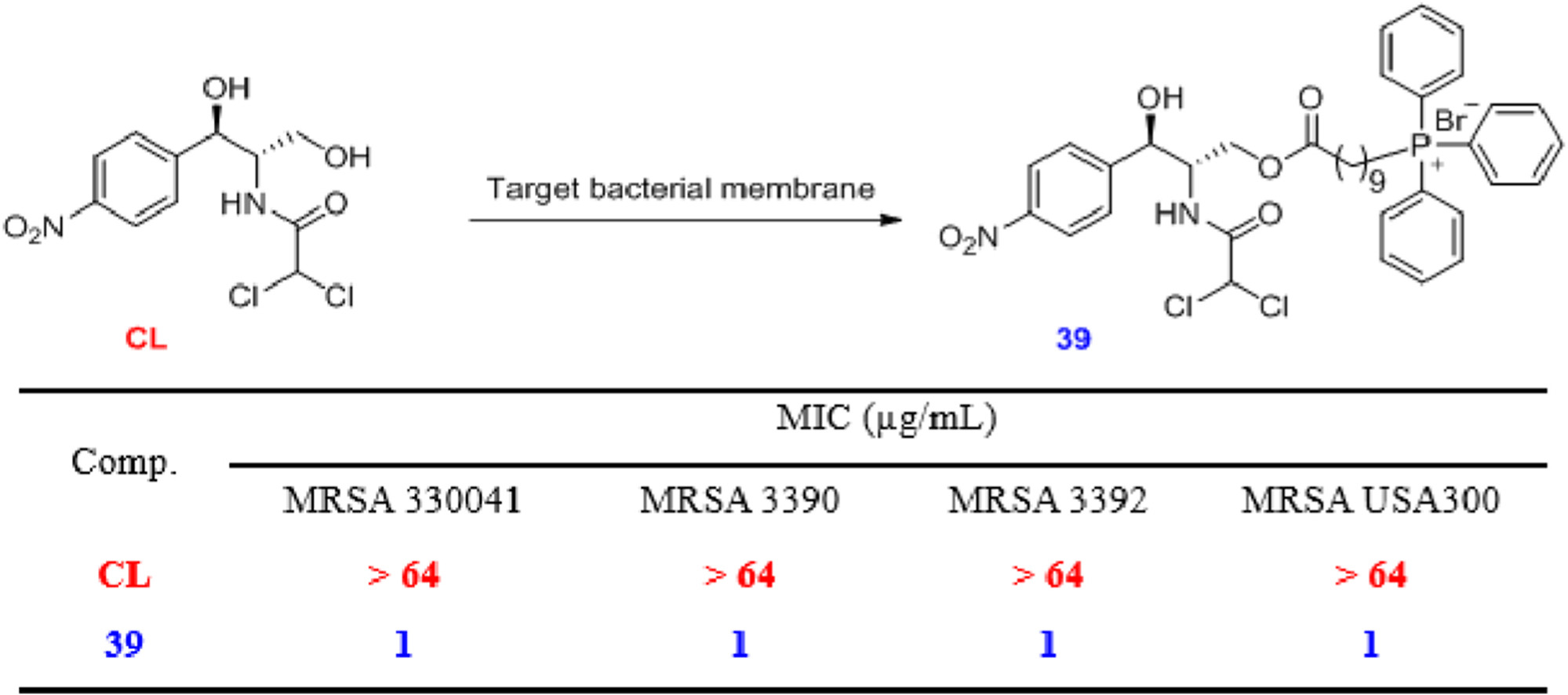
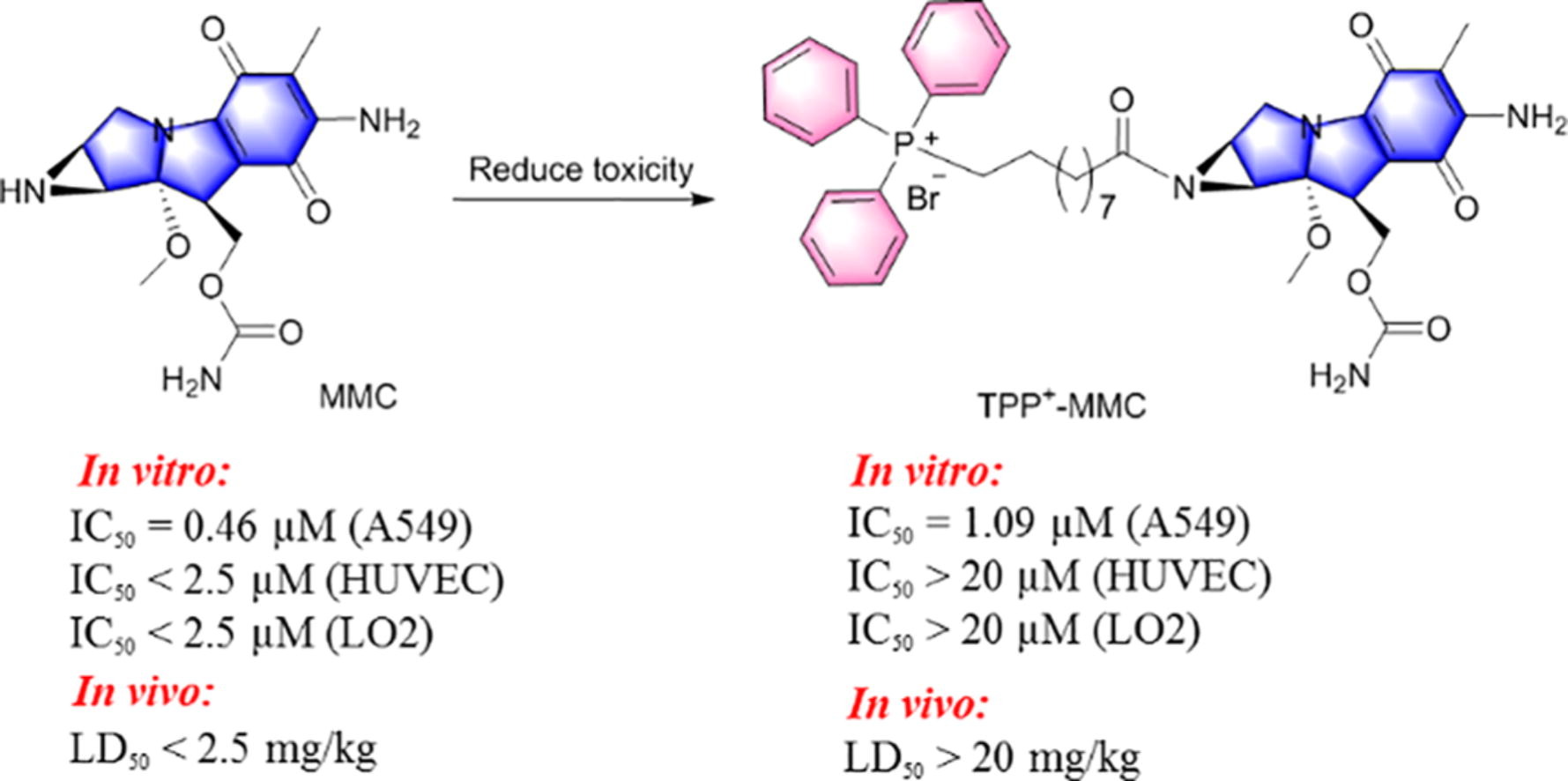
153. T. Li, X. L. He, W. L. Tao, R. X. Zhang, Q. L. He, H. Z. Gong, Y. Liu, D. Luo, M. J. Zhang, C. Zou, Development of membrane-targeting TPP+-chloramphenicol conjugates to combat methicillin-resistant staphylococcus aureus (MRSA) infections, Eur. J. Med. Chem., 2023, 261, 115973. DOI:10.1016/j.ejmech.2023.115973
152. Z. Y. Tang, W. Q. Jiang, S. L. Li, X. Huang, Y. Yang, X. R. Chen, J. Y. Qiu, C. Y. Xiao, Y. Xie, X. Zhang, Design and evaluation of tadpole-like conformational antimicrobial peptides, Commun. Biol, 2023, 6, 1177. DOI: 10.1038/s42003-023-05560-0
151. Z. Y. Tang, J. Z. Feng, S. R. Rowthu, C. Zou, H. B. Peng, C. Huang, Y. He, Uncovering the anti-biofilm activity of Ilicicolin B against Staphylococcus aureus, Biochem. Bioph. Res. Co, 2023, 684, 149138. DOI: 10.1016/j.bbrc.2023.149138
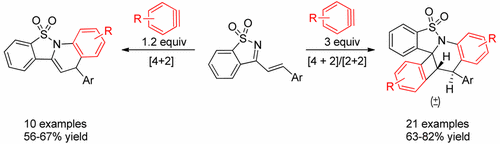
150. L. Zhong, Q. L. Wang, Y. J. Wang, Y. Y. Cheng, Y. M. Xiong, H. B. Peng, Z. Zhou, Y. He, Y. W. Dai, Facile and Stereospecific Synthesis of Diverse β-N-Glycosyl Sulfonamide Scaffolds via Palladium Catalysis, Chem. Commun., 2023, 59, 12907-12910. DOI: 10.1039/D3CC04063A
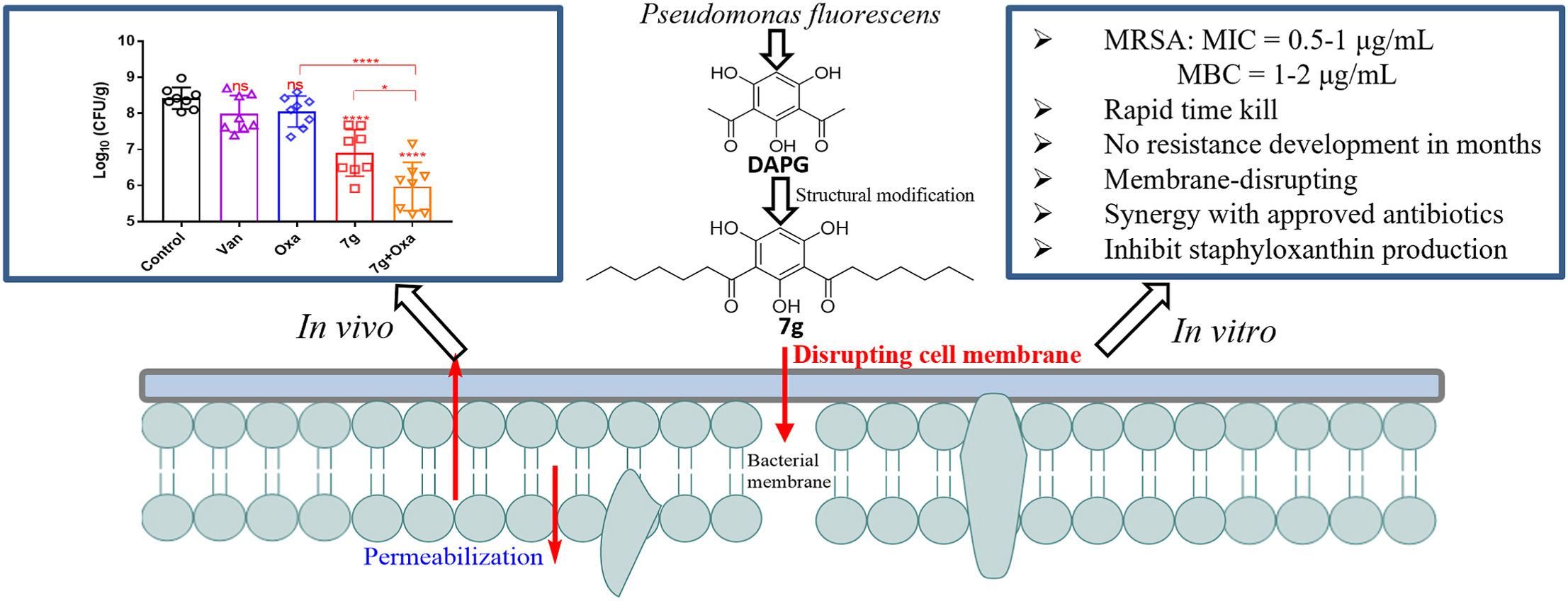
149. Y. F. Zhong, X. L. He,W. L. Tao, J. Z. Feng, R. X. Zhang, H. Z. Gong, Z. Y. Tang, C. Huang, Y. He, 2, 4-Diacetylphloroglucinol (DAPG) derivatives rapidly eradicate methicillin-resistant staphylococcus aureus without resistance development by disrupting membrane, Eur. J. Med. Chem., 2023, 261, 113225. DOI:10.1016/j.ejmech.2023.115823
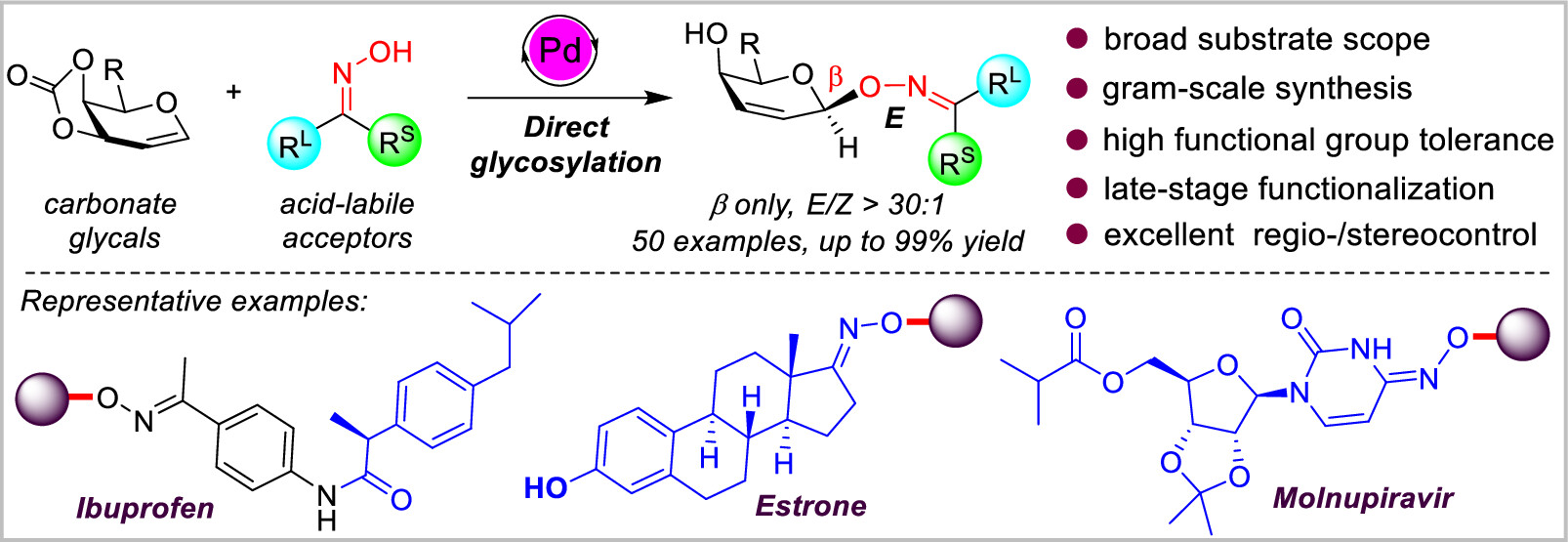
148. Y. J. Wang, Y. Y. Cheng, L. Zhong, S. H. Lei,Y. He, Y. W. Dai, Stereospecific Palladium-Catalyzed Direct Glycosylation of Oximes: Access to N−O-Linked Glycosides. Org. Lett., 2023, 25, 4177-4182. DOI: 10.1021/acs.orglett.3c01484
147. A. Nazli, J. Y. Qi, Z. Y. Tang, Y. He, Recent Advances and Techniques for Identifying Novel Antibacterial Targets, Curr. Med. Chem., 2024,31464-501. DOI: 10.2174/0929867330666230123143458
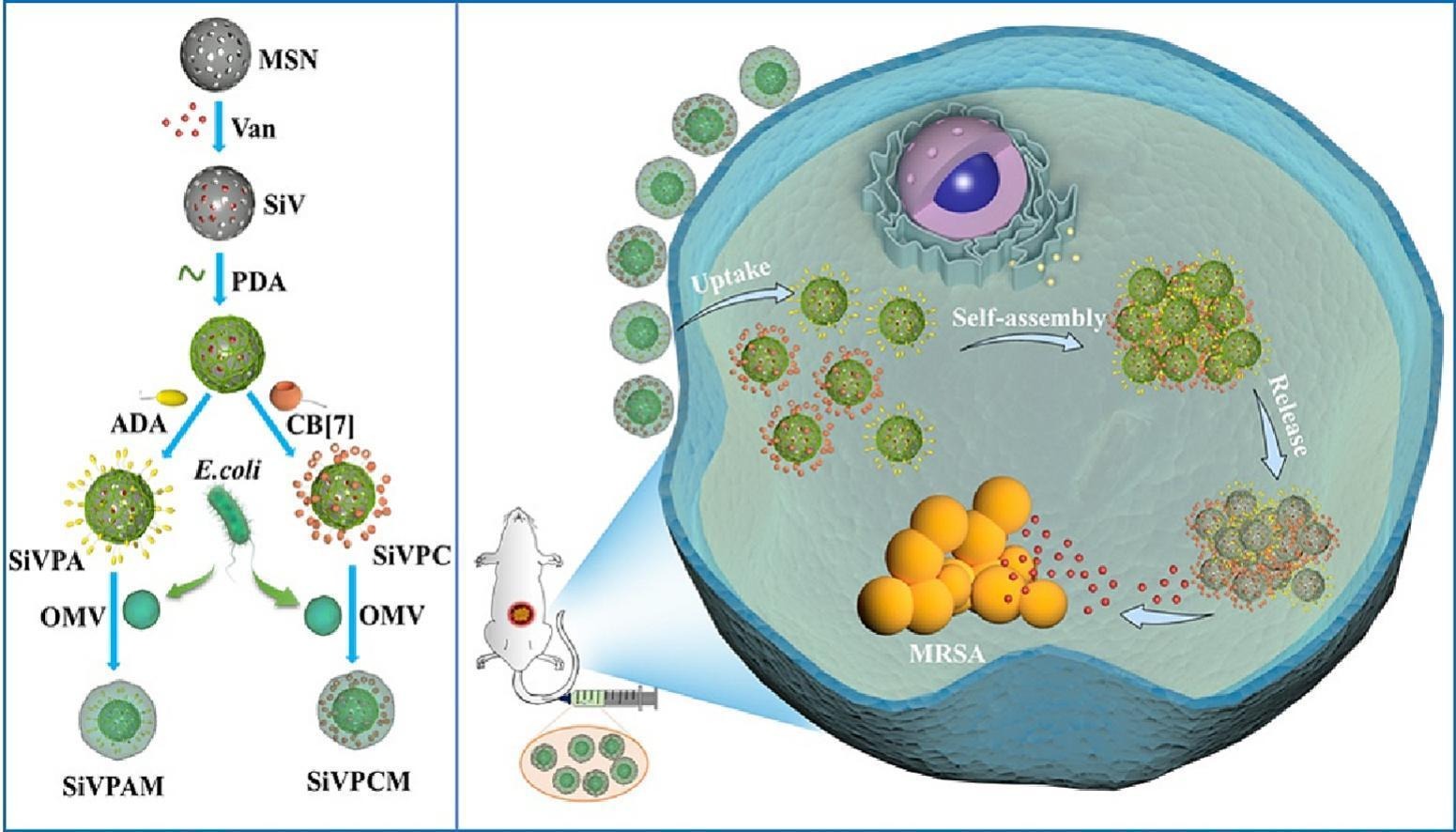
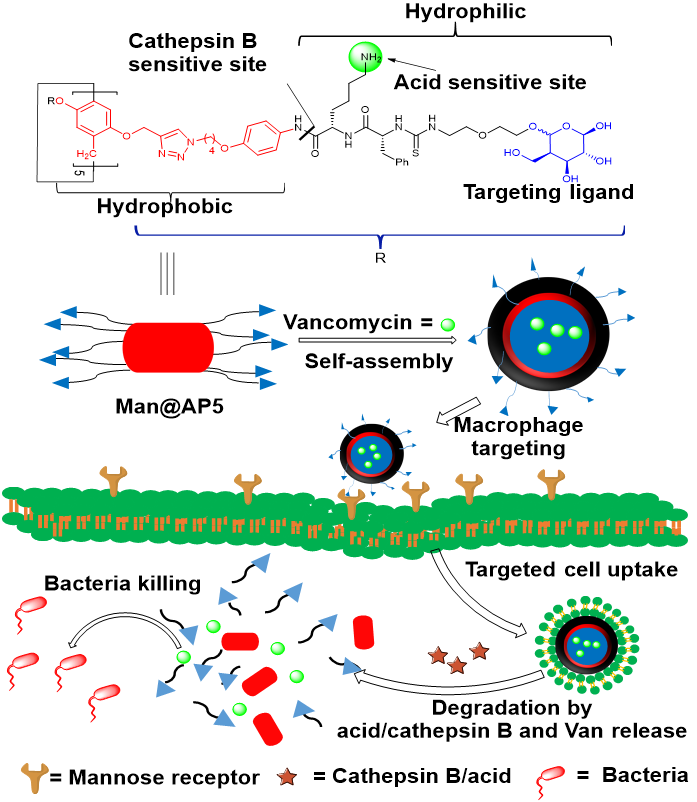
146. B. B. Xie, H. C. Zhao, R. X. Zhang, Y. F. Ding, Cheng, Y. He, R. B. Wang, Bacteria-mimetic nanomedicine for targeted eradication of intracellular MRSA, J. Control. Release., 2023, 357. DOI:10.1016/j.jconrel.2023.03.053
143. S. Zhao, Z. P. Wang, Z. Lin, G. X. Wei, X. M. Wen, S. Y. Li, X. H. Yang, Q. Zhang, C. M. Jing, Y. W. Dai, J. Guo, Y. He, Drug Repurposing by Siderophore Conjugation: Synthesis and Biological Evaluation of Siderophore-Methotrexate Conjugates as Antibiotics, Angew. Chem. Int. Ed. 2022. 134. e202204139 , DOI:10.1002/ange.202204139
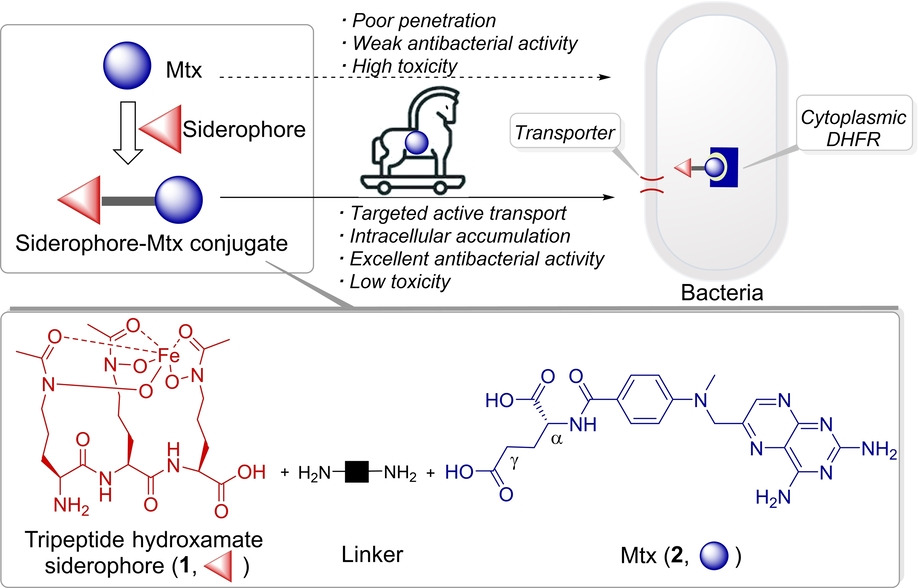
142. D. Huang, Q. W. Liu, M. J. Zhang, Y. Z. Guo,Z. Y. Cui, T. Li, D. Luo, B. Xu,C. Huang,J. Guo, Jian. K. Y. Tam, M. Zhang, S. L. Zhang, Y. He, A Mitochondria-Targeted Phenylbutyric Acid Prodrug Confers Drastically Improved Anticancer Activities, J. Med. Chem, 2022. 65. 14. 9955-9973. DOI:10.1021/acs.jmedchem.2c00640
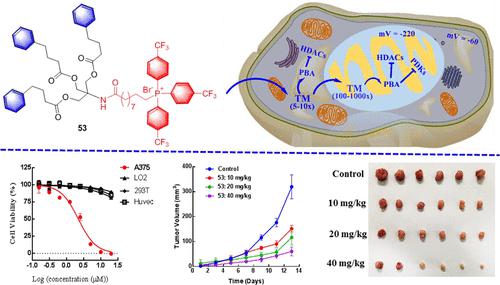
141. X. H. Yang, Z. P. Wang, S. C. Xiang, D. Q. Wang, Y. Zhao, D. Luo, Y. F. Qiu, C. Huang, J. Guo, Y. W. Dai, S. L. Zhang, Y. He, Optimization of the Natural Product Calothrixin A to Discover Novel Dual Topoisomerase I and II Inhibitors with Improved Anticancer Activity, J. Med. Chem, 2022, 65. 11, 8040-8061. DOI: 10.1021/acs.jmedchem.2c00615
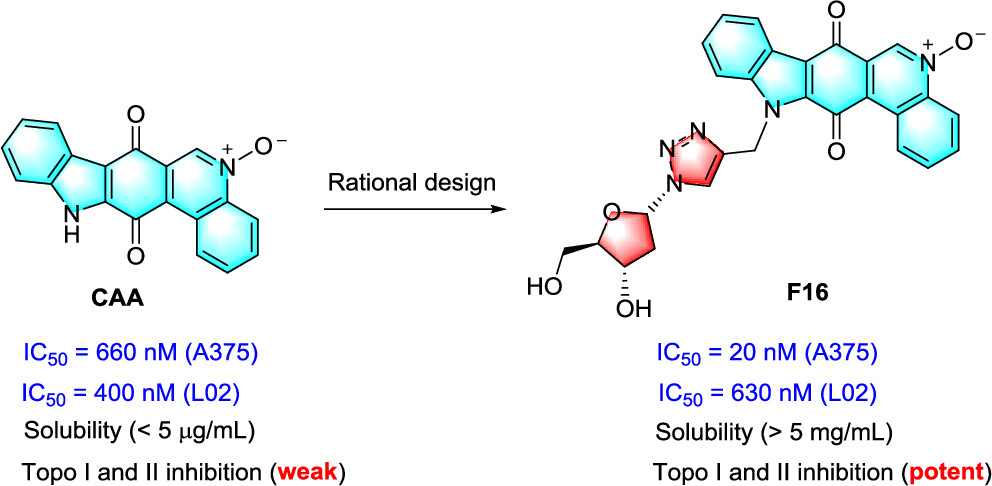
140. C. P. Liu, J. X. Han, O. Marcelina, D. Nugrahaningrum, S. Huang, M. J. Zou, G. X. Wang, M. Makoto,Y. He,S. R. Wu, V. Kasim, Discovery of Salidroside-Derivated Glycoside Analogs as Novel Angiogenesis Agents to Treat Diabetic Hind Limb Ischemia, J. Med. Chem, 2022, 65, 1, 135-162. DOI: 10.1021/acs.jmedchem.1c00947.
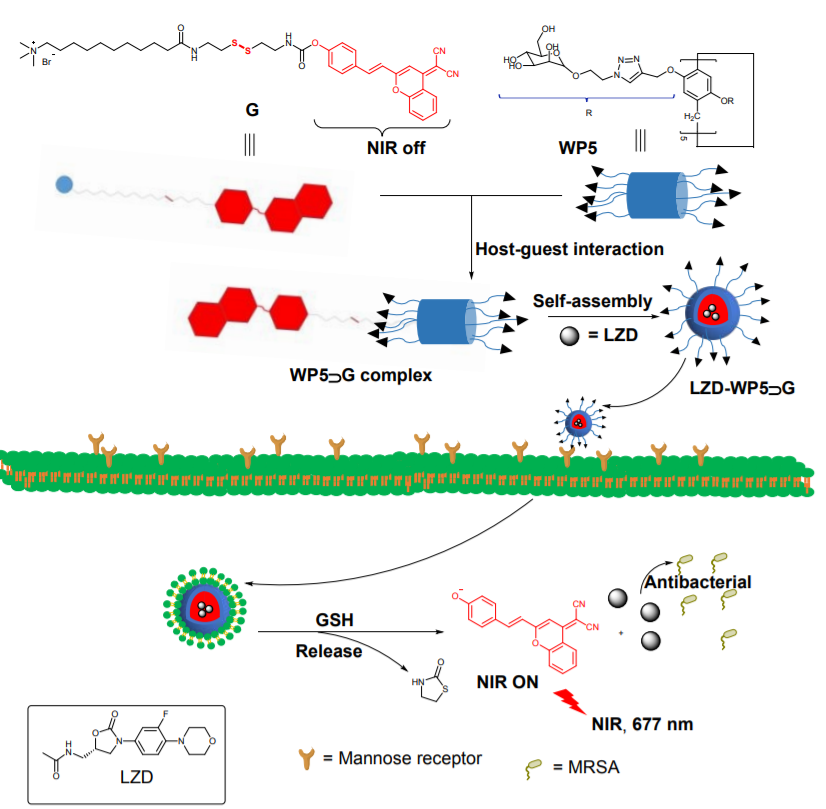
139. H. B. Peng, B. B. Xie, J. J. Dai, Y. W. Dai, X. F. Cen, X. H. Yang, Y. He, GSH-Responsive Multifunctional Nanoparticles Based on Mannose-Modified Pillar[5]arene for Targeted Antibiotic Delivery against Intracellular MRSA. Mater. Chem. Front., 2022, 6, 360 , DOI: 10.1039/D1QM01459E
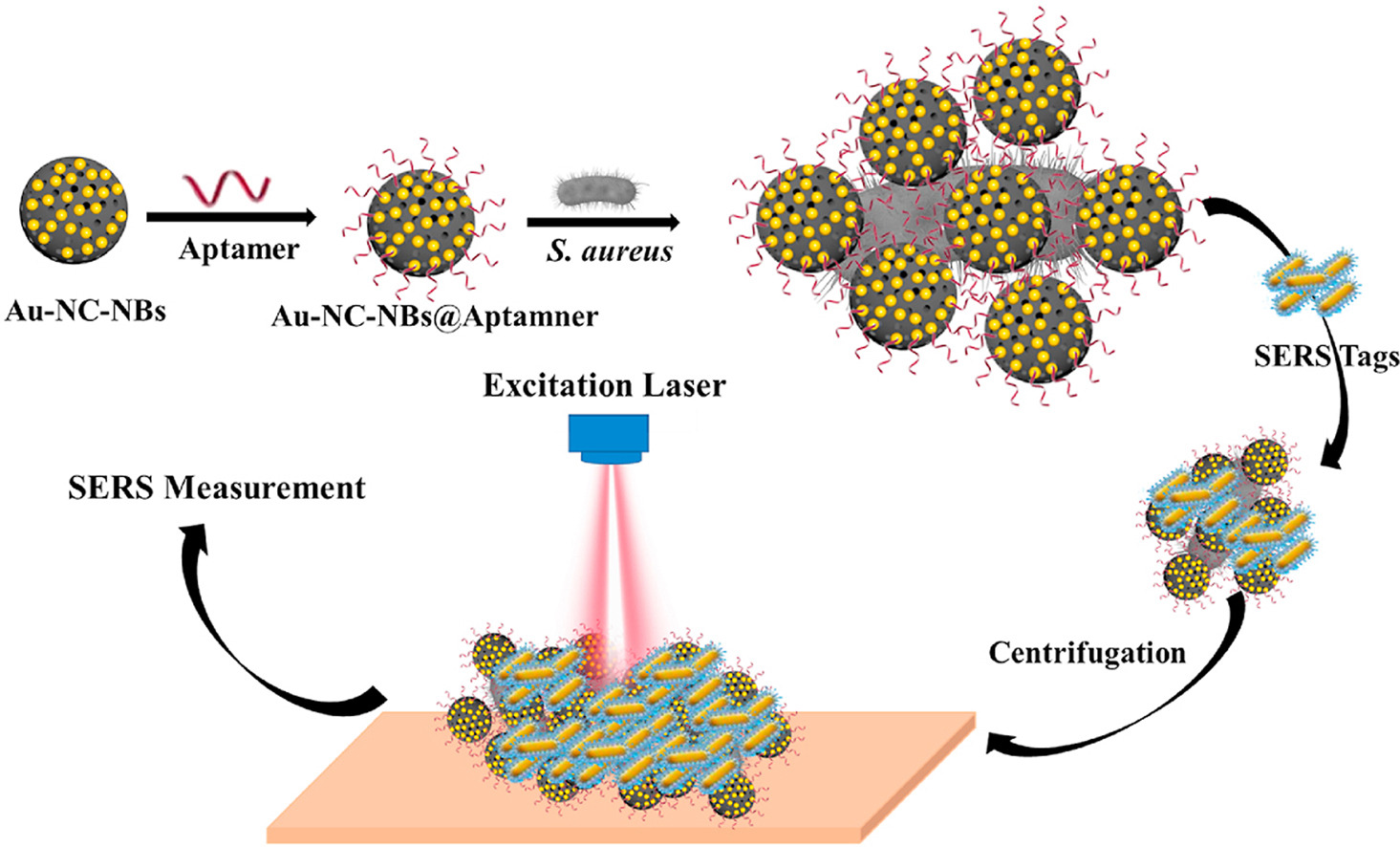
138. B. B. Xie, Z. P. Wang, R. X. Zhang, Z. Zhang, Y. He, A SERS Aptasensor based on Porous Au-NC Nanoballoons for Bacteria Detection. Anal. Chim. Acta., 2022, 1190, 339175. DOI: 10.1016/j.aca.2021.339175
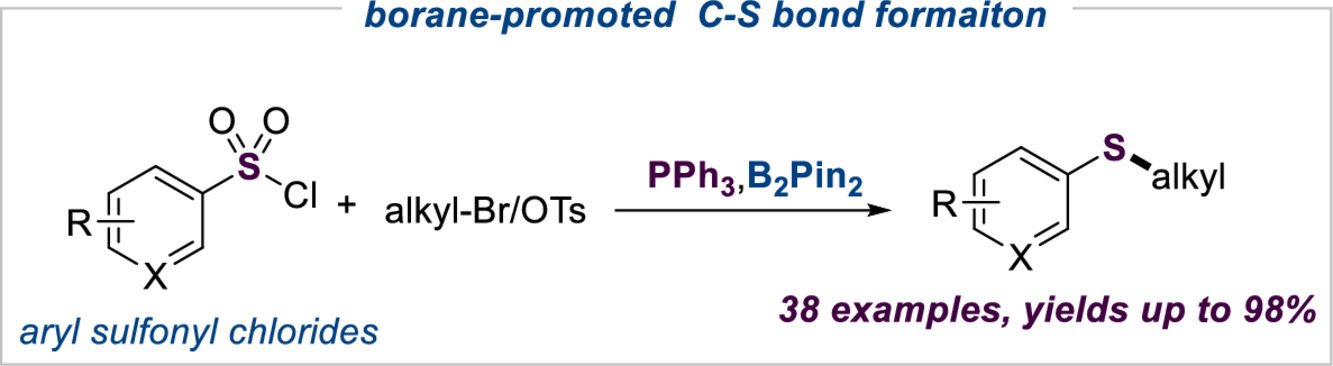
137. S. Chen, Q. R. Wen, Y. Q. Zhu, Y. R. Ji, Y. Pu,Z. L. Liu, Y. He, Z. Feng, Boron-promoted reductive deoxygenation coupling reaction of sulfonyl chlorides for the C (sp3)-S bond construction. Chinese. Chem. Lett., 2022, 33, 12, 5101. DOI: 10.1016/j.cclet.2022.04.022
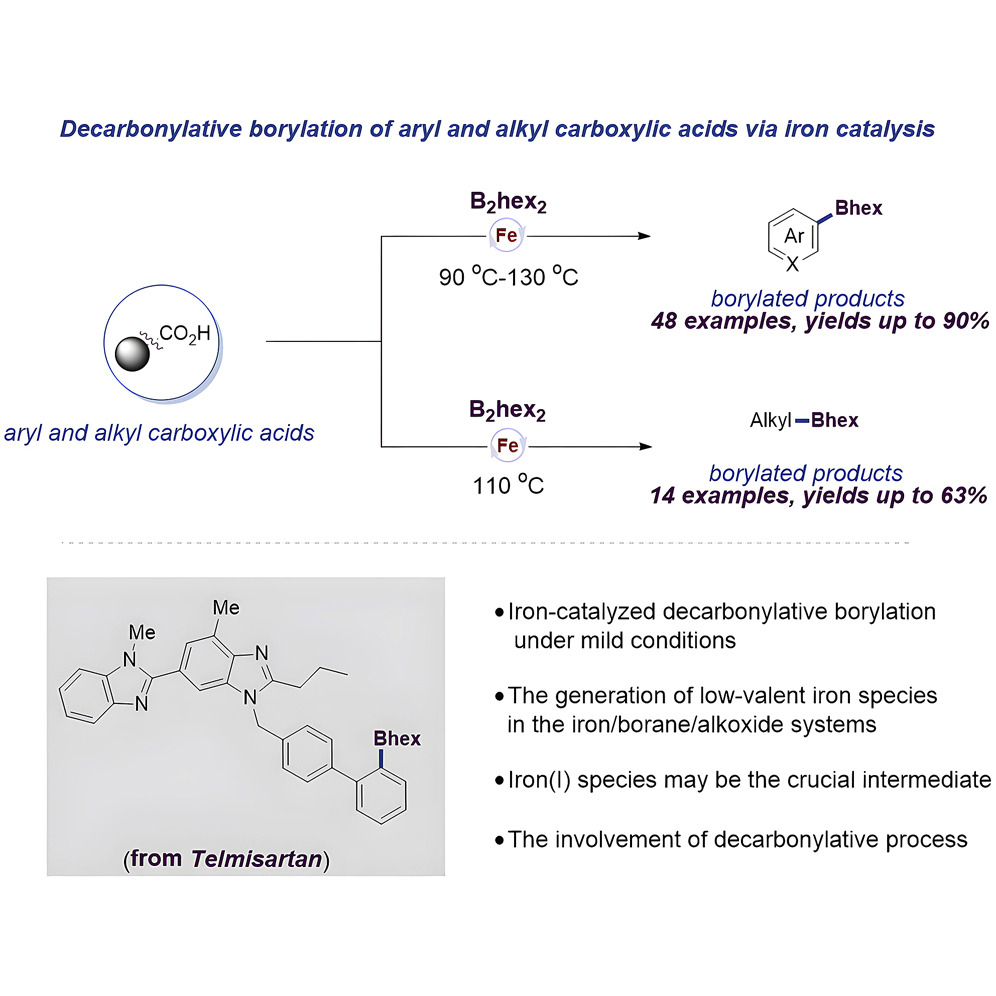
136. Q. R. Wen, S. Chen, C. Q. Shi, S. Chen,Y. R. Ji, J. Guo,Z. L. Liu, Y. He, Z. Feng, Iron-catalyzed decarbonylative borylation enables the one-pot diversification of (Hetero) Aryl and alkyl carboxylic acids. Cell. Rep. Phys. Sci., 2022, 3, 8, 100995. DOI: 10.1016/j.xcrp.2022.100995
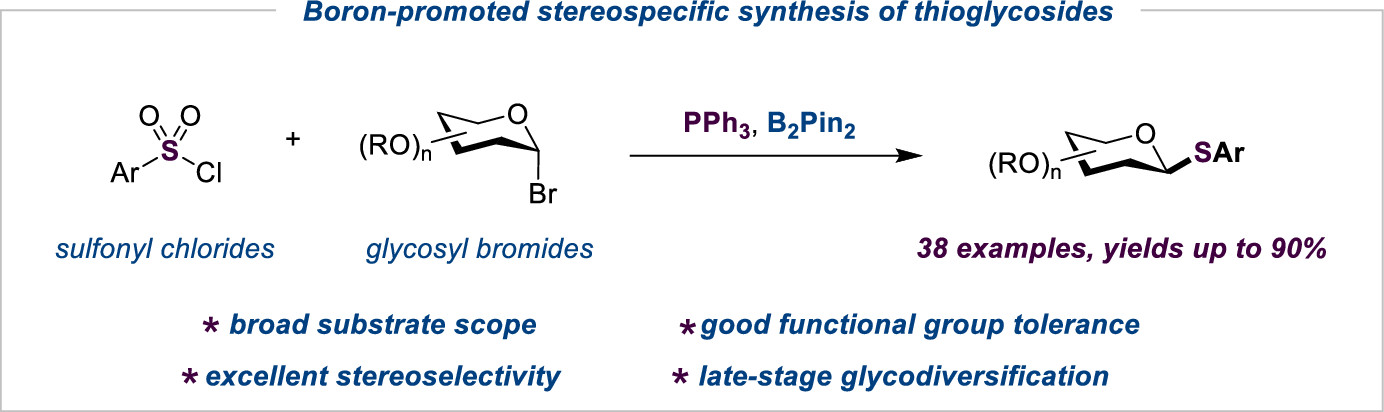
135. S. Y. Li, Y. J. Wang, L. Zhong, S. Y. Wang, Z. L. Liu, Y. W. Dai, Y. He,Z. Feng, Boron-Promoted Umpolung Reaction of Sulfonyl Chlorides for the Stereospecific Synthesis of Thioglycosides via Reductive Deoxygenation Coupling Reactions. Org. Lett., 2022, 24, 13, 2463. DOI: 10.1021/acs.orglett.2c00353
134. A. Nazli, D. He, H. C. Xu, Z. P. Wang, Y. He, A Comparative Insight on the Newly Emerging Rifamycins: Rifametane, Rifalazil, TNP-2092 and TNP-2198, Curr. Med. Chem., 2022, 29. 2846. DOI:10.2174/0929867328666210806114949
133. G. X. Wei, Y. He, Antibacterial and Antibiofilm Activities of Novel Cyclic Peptides against Methicillin-Resistant Staphylococcus aureus, Int. J. Mol. Sci., 2022, 23. 8029. DOI:10.3390/ijms23148029
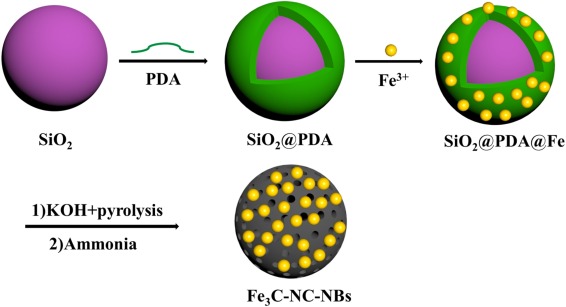
132. B. B. Xie, X. H. Yang, R. X. Zhang, J. Guo, Z. C. Chen and Y. He, Hollow and Porous Fe3C-NC Nanoballoons Nanozymes for Cancer Cell H2O2 Detection, Sens. Actuators B Chem., 2021, 347, 130579, DOI: 10.1016/j.snb.2021.130597
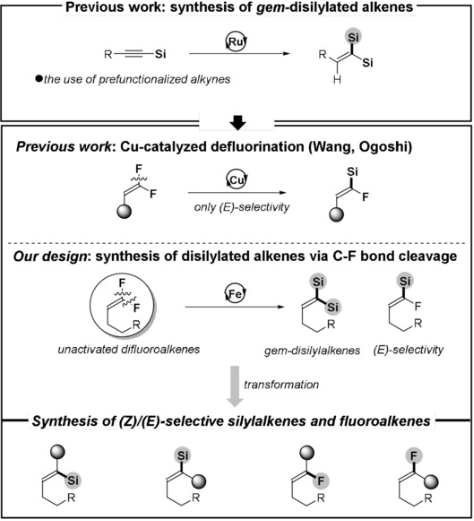
131. H. Zhang, E. Wang, S. Geng, Z. Liu, Y. He, Q. Peng, Z. Feng, Experimental and Computational Studies of the Iron‐Catalyzed Selective and Controllable Defluorosilylation of Unactivated Aliphatic gem‐Difluoroalkenes. Angew. Chem. Int. Ed., 2021, 60, 10211-10218. DOI:10.1002/anie.202100049
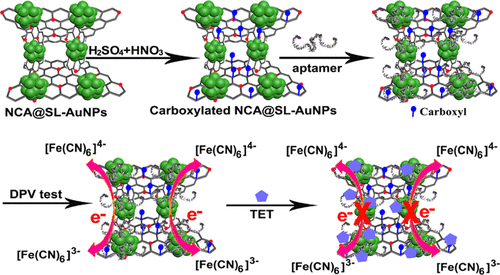
130. B. B. Xie, H. B. Peng, R. X. Zhang, C. H. Wang, Y. He, A Label-Free Electrochemical Aptasensor Based on Stone-Like Gold Nanoparticles for Ultrasensitive Detection of Tetracycline, J. Phys. Chem. C., 2021, 125. 5678-5683. DOI:10.1021/acs.jpcc.0c10809
129. B. Xu, Z. P. Wang, Q. W. Liu, X. H. Yang, X. M. Li, D Huang, Y. F. Qiu, K. Y. Tam, S. L. Zhang and Y. He, Synthesis, biological evaluation and structure-activity relationship of novel dichloroacetophenones targeting pyruvate dehydrogenase kinases with potent anticancer activity, Eur. J. Med. Chem., 2021, 214, 113225. DOI:10.1016/j.ejmech.2021.113225
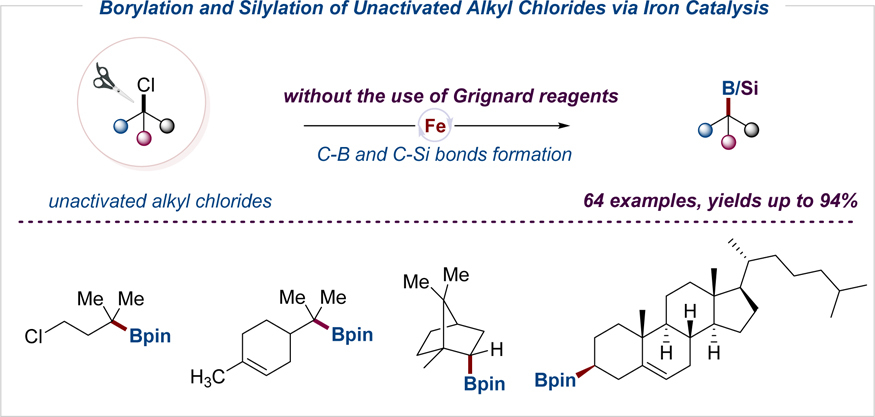
128. S. Y. Wang, M. H. Sun, H. Zhang, J. Zhang, Y. He, Z. Feng, Iron-Catalyzed Borylation and Silylation of Unactivated Tertiary, Secondary, and Primary Alkyl Chlorides, CCS. Chem, 2021, 3, 9, 2164. DOI:10.31635/ccschem.020.202000447
127. H. Y. Qiu, R. P. Clausen,Y. He, H. L. Zhu, Artificial Intelligence and Cheminformatics-Guided Modern Privileged Scaffold Research, Curr. Top. Med. Chem., 2021, 28, 21, 2593. DOI:10.2174/1568026621666210512020434
126. P. F. Wang, Y. F. Qiu, Y. He, H. L. Zhu, Cyclin-dependent kinase 4/6 inhibitors for cancer therapy: a patent review (2015–2019), Expert. Opin. Ther. Pat., 2020, 30. 795-805. DOI:10.1080/13543776.2020.1825686
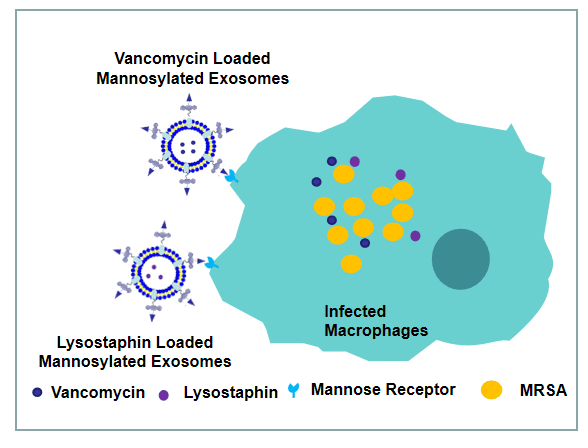
125. X. H. Yang, B. B. Xie, H. B. Peng, G. M. Shi, B. Sreenivas, Y. F. Qiu, C. H. Wang, Y. He, Mannose-modified Exosomes for Targeted Delivery of Lysostaphin and Vancomycin to Eradicate Intracellular MRSA, J. Control. Release, 2020, 329, 454-467.DOI:10.1016/j.jconrel.2020.11.045
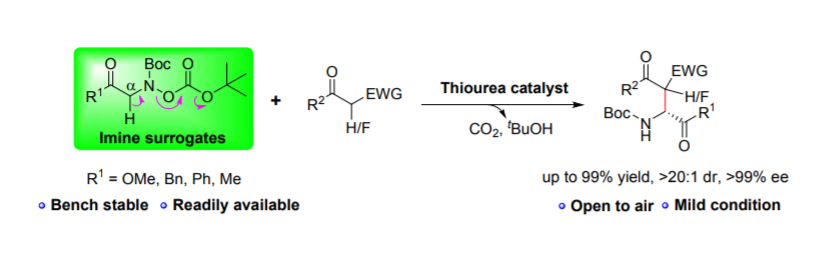
124. H. C. Xu, A. Nazli, Z. P. Wang, Y. He, Bench-stable Imine Surrogates for the Efficient, One-pot and Catalytic Asymmetric Synthesis of α-Amino Esters/Ketones, Chem. Comm., 2020, 56, 14243-14246, DOI: 10.1039/D0CC06055K
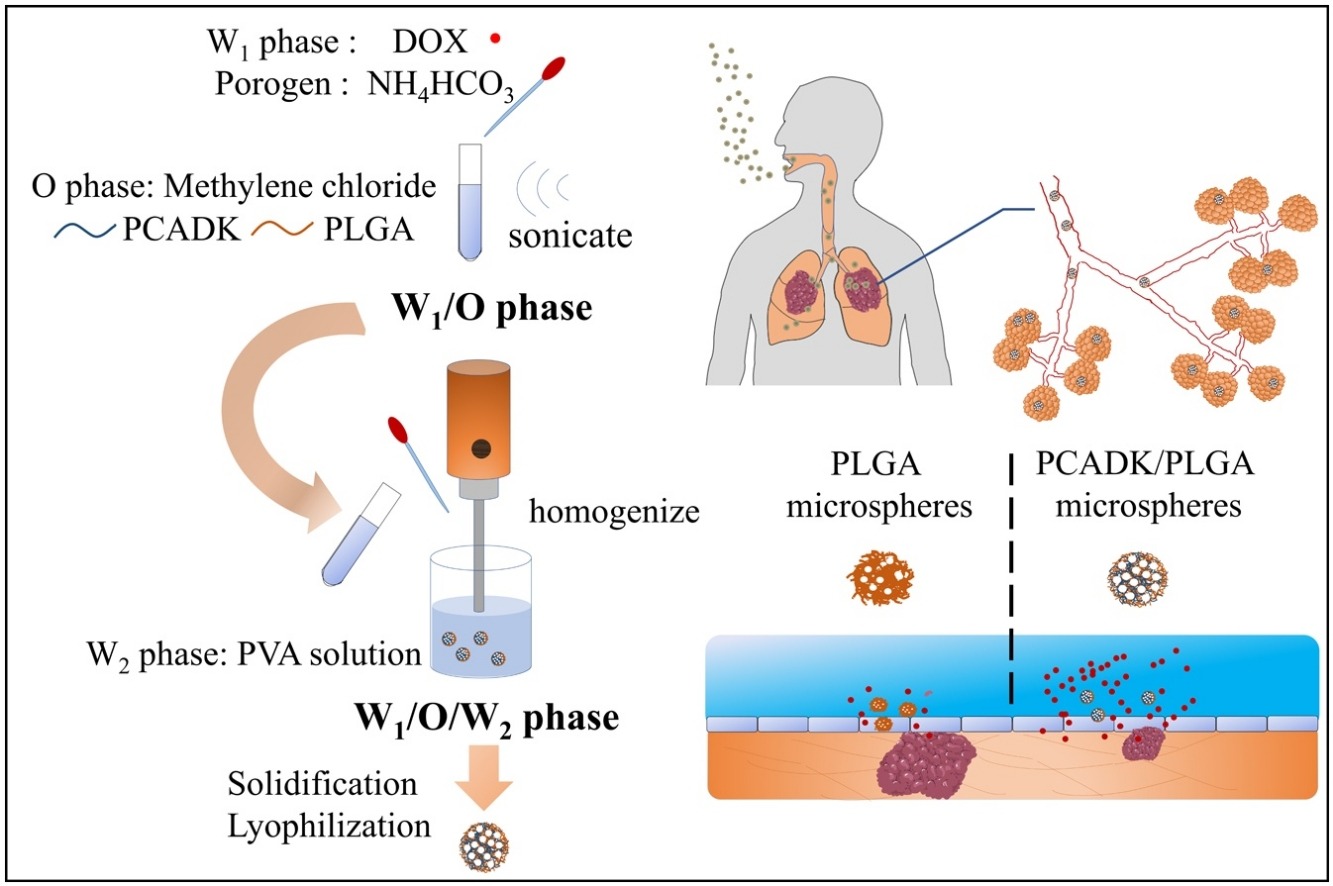
123.W. X. Li, S. Chen, L. F. Zhang, Y. Zhang, X. H. Yang, B. B. Xie, J. Guo, Y. He, C. H. Wang, Inhalable functional mixed-polymer microspheres to enhance doxorubicin release behavior for lung cancer treatment, Colloid Surface B., 2020, 111350. DOI: 10.1016/j.colsurfb.2020.111350
122. S. Zhao, Z. P. Wang, X. M. Wen, S. Y. Li, G. X. Wei, J. Guo, Y. He, Synthesis of Vitamin B12-Antibiotic Conjugates with Improved Activity Against Gram-Negative Bacteria, Org. Lett., 2020, 16, 6632–6636, DOI:10.1021/acs.orglett.0c02403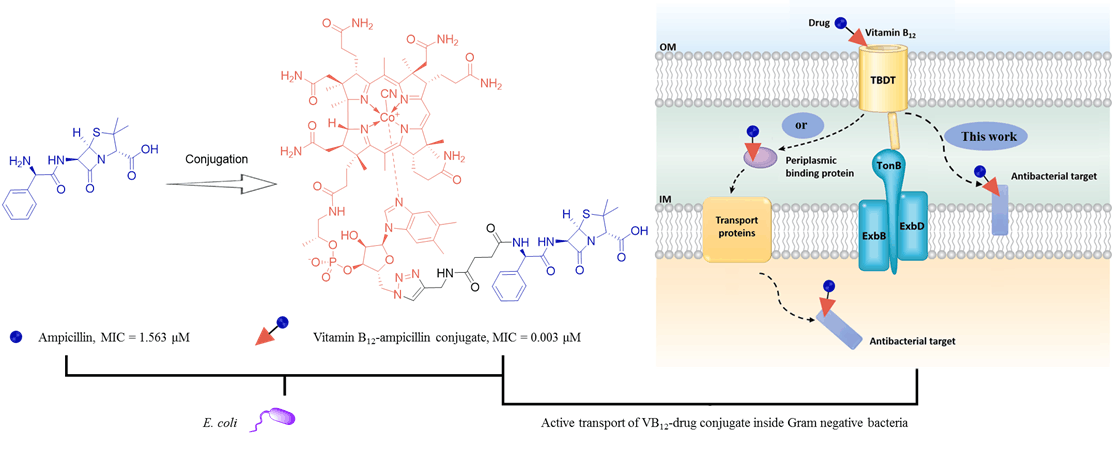
121. X. Zeng, Y. Zhang, Z. Liu, S. Geng, Y. He, Z. Feng, Iron-Catalyzed Borylation of Aryl Ethers via Cleavage of C–O Bonds, Org. Lett., 2020, 22, 2950-2955. DOI:10.1021/acs.orglett.0c00679

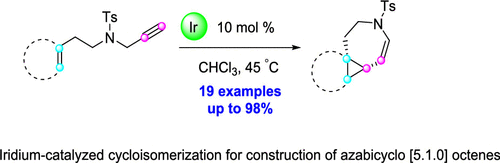
120. S. Zhao, Z. L. Zhang, S. Y. Li, X. M. Wen, J. Guo, Y. He, Iridium-Catalyzed Cycloisomerization of N-Tethered 1,7-Enynes: Construction of Azabicyclo [5.1.0] octene System, J. Org. Chem., 2020. 85, 9321-9330. DOI:10.1021/acs.joc.0c00516
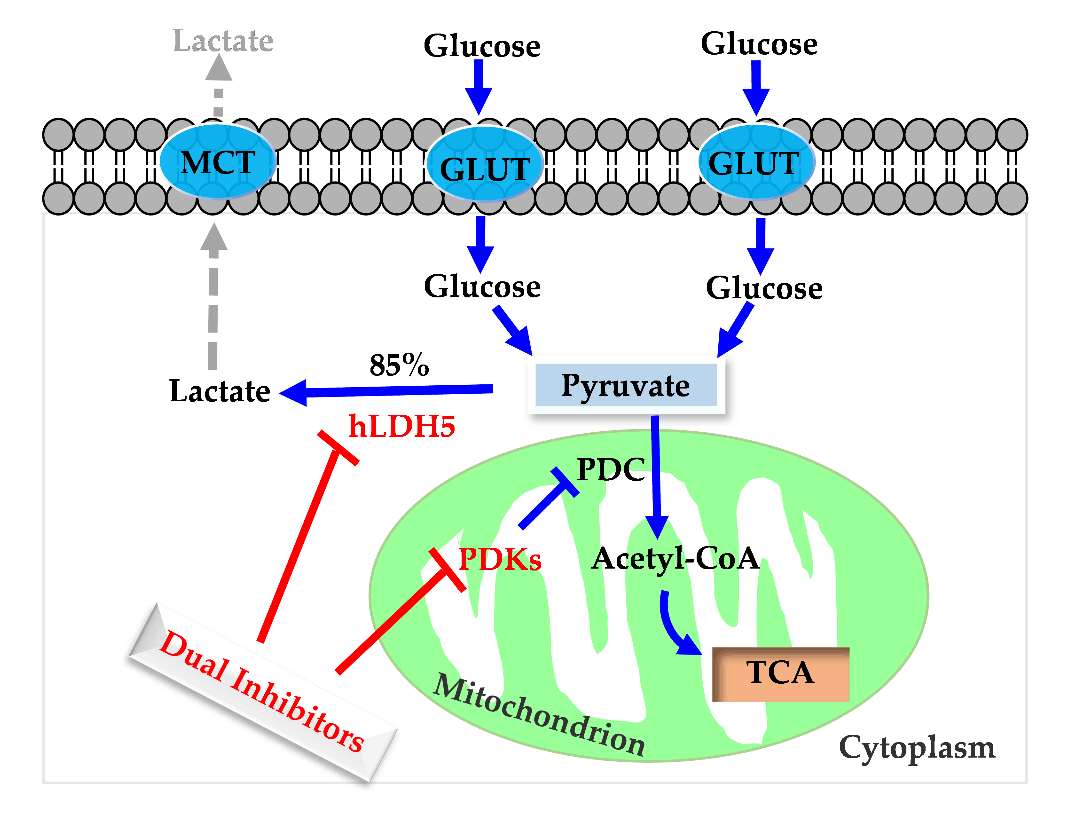
119. S. C. Xiang, D. Huang, Q. L. He, J. Li, K. Y. Tam, S. L. Zhang, Y. He, Development of Dual Inhibitors Targeting Pyruvate Dehydrogenase Kinases and Human Iactate Dehydrogenase A: High-throughput Virtual Screening, Synthesis and Biological Validations, Eur. J. Med. Chem., 2020, 1, 112579. DOI:10.1016/j.ejmech.2020.112579
118. H. B. Peng, B. B. Xie, X. H. Yang, J. J. Dai, G. X. Wei, Y. He, Pillar[5]arene-based, Dual pH and Enzyme Responsive Supramolecular Vesicles for Targeted Antibiotic Delivery Against Intracellular MRSA, Chem. Commun., 2020, 56, 8115-8118. DOI: 10.1039/D0CC02522D
117. B. B. Xie, H. B. Peng, C. H. Wang, Z. Zhang, Y. He, Controlled Coassembly of Dumbbell-like Au Nanoparticles with a Porous Nitrogen Doped Carbon Aerogel for Cancer Cell H2O2 Detection, Anal. Chim. Acta., 2020. 22.100-105.DOI:10.1016/j.aca.2020.06.003

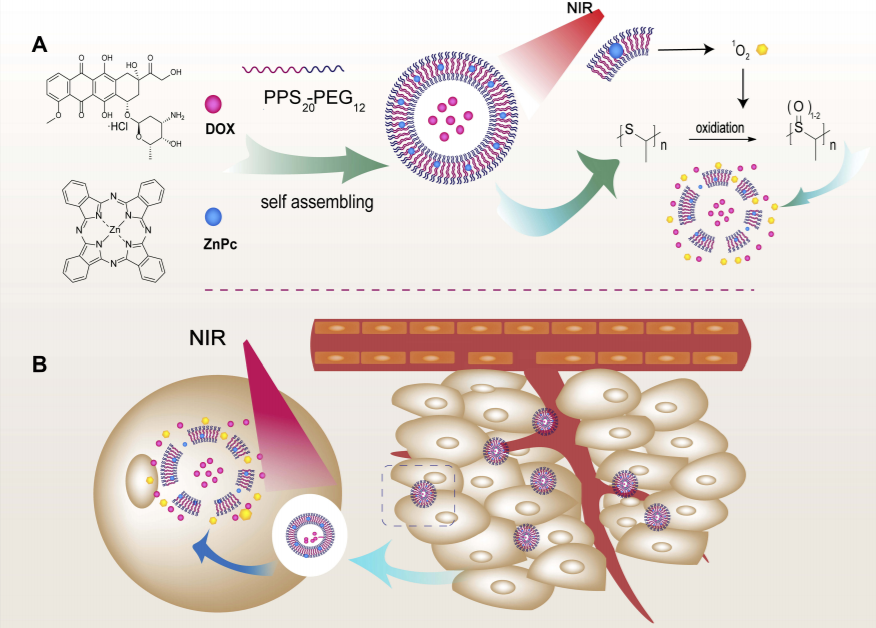
116. Q. Tang, P. Hu, H. Peng, N Zhang, Q Zheng, Y. He, Near-Infrared Laser-Triggered, Self-immolative Smart Polymersomes for in Vivo Cancer Therapy, Int. J. Nanome. 2020., 15, 137-149. DOI: 10.2147/IJN.S224502
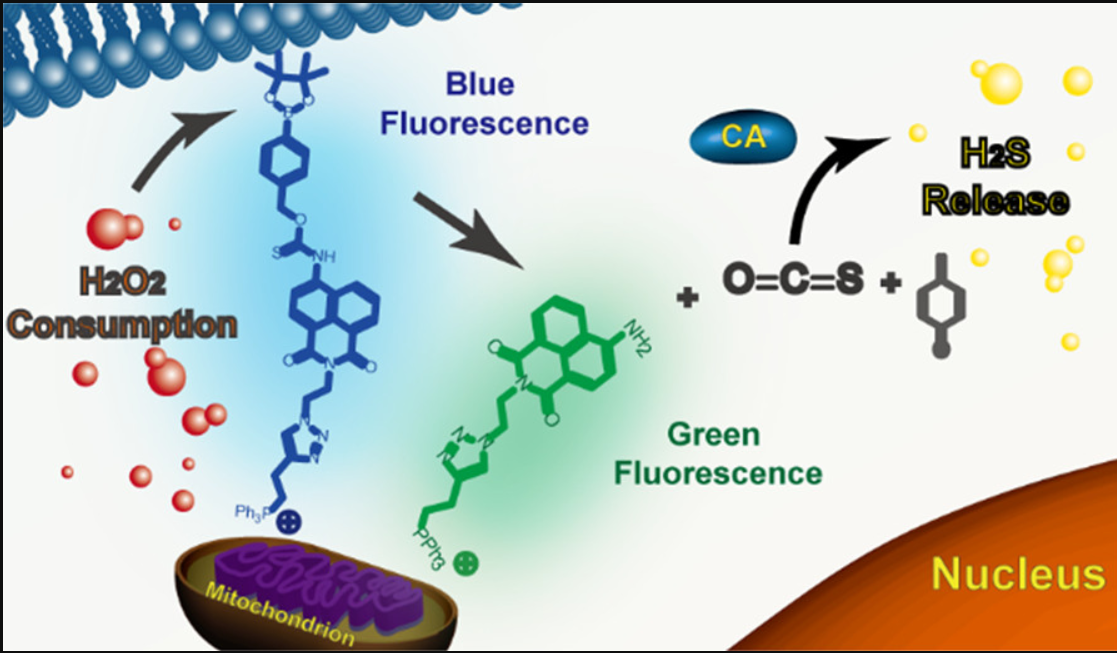
115. N. Zhang, P Hu, Y Wang, Q. Tang, Q. Zheng, Z. Wang, Y. He, A Reactive Oxygen Species (ROS) Activated Hydrogen Sulfide (H2S) Donor with Self-Assemblyreporting Fluorescence, ACS sensors., 2020, 5, 319-326. DOI : 10.1021/acssensors.9b01093
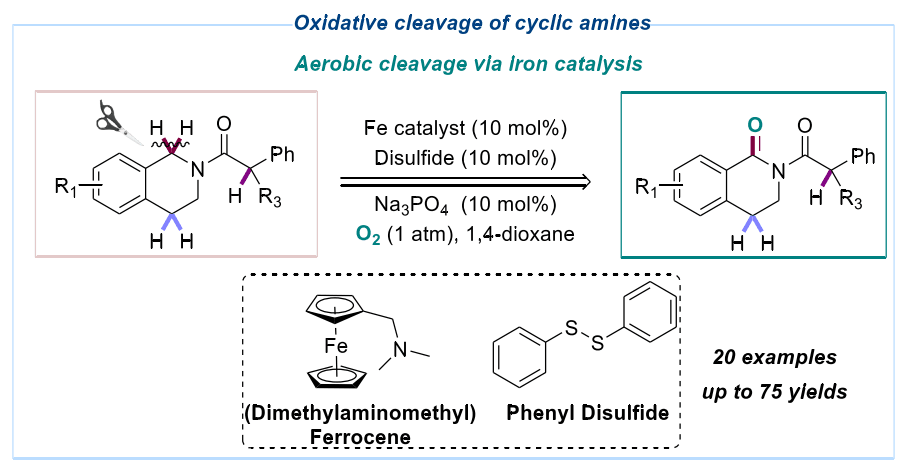
114. S. S. Geng, B. J. Xiong, Y Zhang, J Zhang, Y He, Z. Feng, Thiyl Radical Promoted Iron-catalyzed-selective Oxidation of Benzylic sp3 C–H Bonds with Molecular Oxygen, Chem. Commun., 2019, 55 (84), 12699-12702. DOI: 10.1039/C9CC06584A
113. C. L. Mao, S. Zhao, Z. L. Zang, L. Xiao, C. H. Zhou, Y. He and G. X. Cai, Pd-Catalyzed Remote Site-selective and Stereoselective C(Alkenyl)− H Alkenylation of Unactivated Cycloalkenes, J. Org. Chem., 2019, 85, 774-787. DOI: 10.1021/acs.joc.9b02797
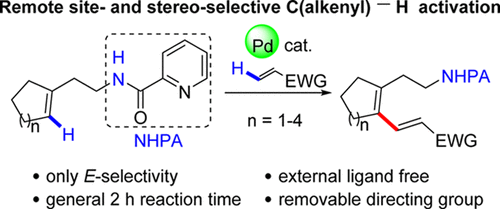
112. Q. Wu, P. L. Shao, Y. He, Synthesis of 1,4,5,6-Tetrahydropyridazines and Pyridazines via Transition-Metal-Free (4+2) Cycloaddition of Alkoxyallenes with 1,2-Diaza-1,3-Dienes, RSC Advances., 2019, 9, 21507–21512. DOI: 10.1039/c9ra02712b
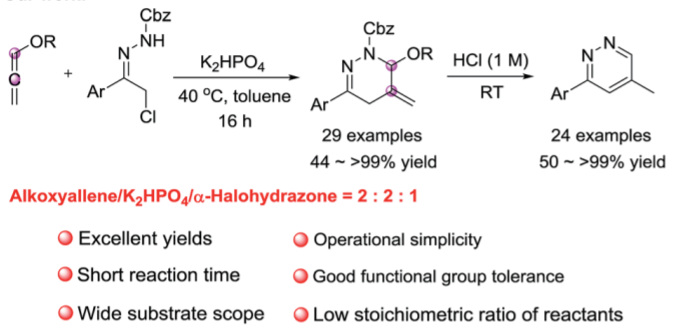
111. L. Chandraiah, B. Sreenivas, H. Y. You, M. Tang, J. Guo, N. Qi, Y. He, The Construction of Bridged-ring-fused Naphthalenone Derivatives Through an Unexpected Zn(OTf)2-Catalyzed Cascade Transformation, Org. Lett., 2019, 21, 13, 5301-5304. DOI: 10.1021/acs.orglett.9b01912
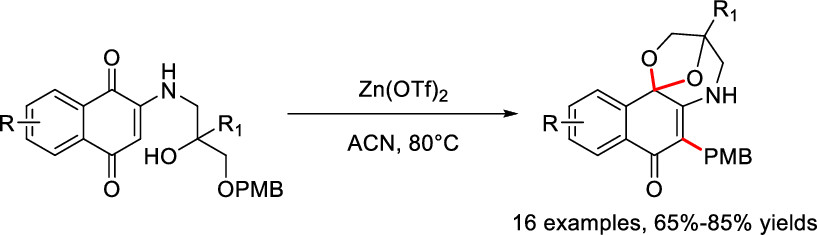
110. A. Srinivasa, B. Sreenivas, J. Jiang, N. Qi, J. Guo, Y. He, A Unified Synthetic Approach to Optically Pure Curvularin-type Metabolites, J. Org. Chem., 2019, 84, 11, 7227-7237. DOI: 10.1021/acs.joc.9b00776
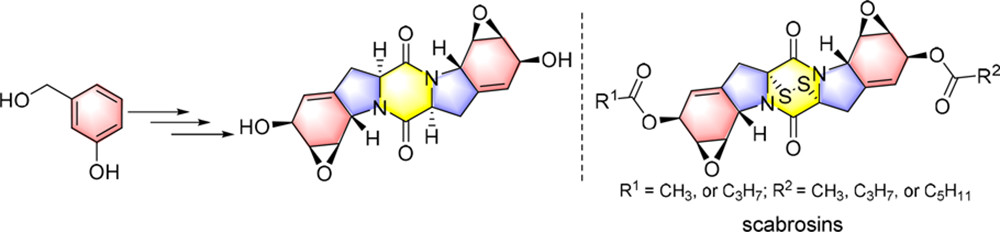
109. Y. Y. Liu, Z. P. Wang, S. Banne, J. Guo, Y. He, Towards the Total Synthesis of Scabrosins: Synthesis of Desulfur-scabrosins Skeleton and Its Stereoisomers, J. Org. Chem., 2019, 84, 5838-5845. DOI: 10.1021/acs.joc.9b00015
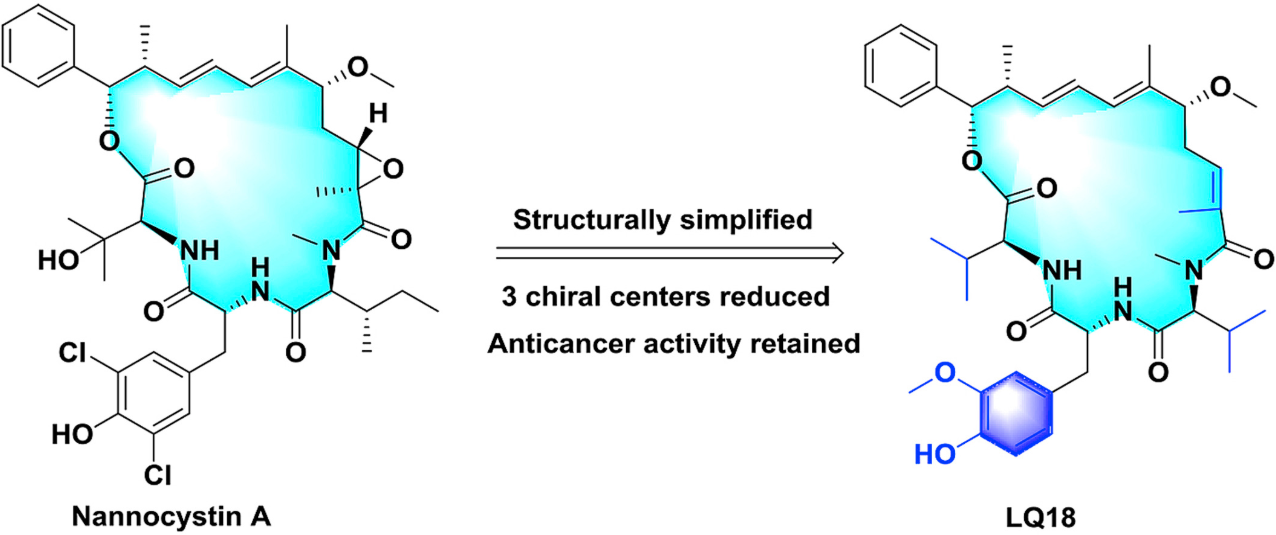
108. Q. Liu, X. H. Yang, J. Ji, S. L. Zhang, Y. He, Novel Nannocystin A Analogues as Anticancer Therapeutics: Synthesis, Biological Evaluations and Structure-activity Relationship Studies, Eur. J. Med. Chem., 2019, 170, 99-111. DOI: 10.1016/j.ejmech.2019.03.011
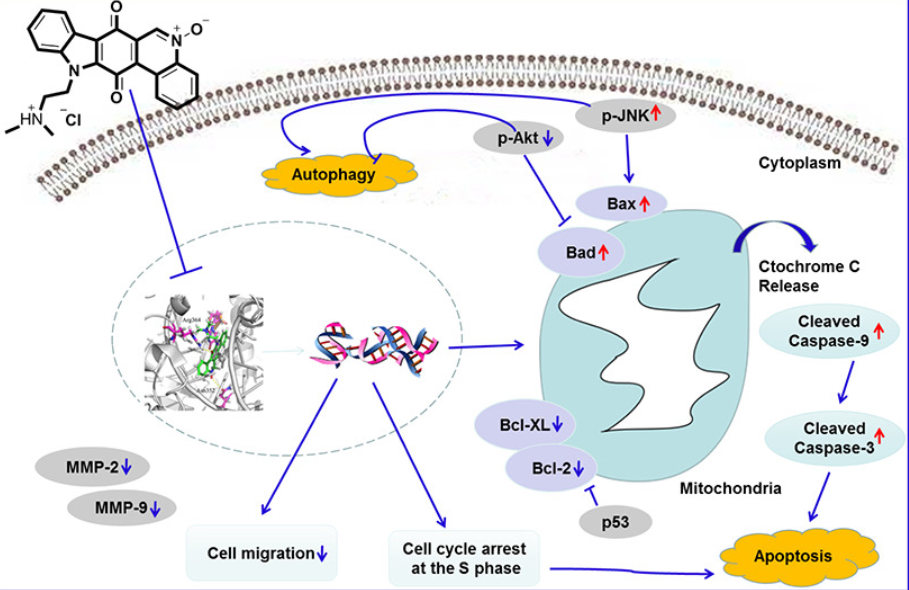
107. X. H. Yang, J. S. Gao, J. Guo, Z. M. Zhao, S. L. Zhang, Y. He, Anti-lung Cancer Aactivity and Inhibitory Mechanisms of a Novel Calothrixin A Derivative, Life Sciences, 2019, 219, 20-30. DOI: 10.1016/j.lfs.2018.12.052
106. Q. Zheng, Y. He, Q. Tang, Y. Wang, N. Zhang, J. Liu, Q. Liu, S. Zhao, P. Hu, An NIR-Guided Aggregative and Self-Immolative Nanosystem for Efficient Cancer Targeting and Combination Anticancer Therapy, Mol. Pharmaceutics, 2018, 15, 4985-4994. DOI: 10.1021/acs.molpharmaceut.8b00599
105. N. Zhang, Y. He, Q. Tang, Y. Wang, Q. Zheng, P. Hu, A Mitochondrial Targeting Two-channel Responsive Fluorescence Probe for Imaging the Superoxide Radical Anion in Vitro and in Vivo, Talanta, 2019, 194, 79-85. DOI: 10.1016/j.talanta.2018.09.109
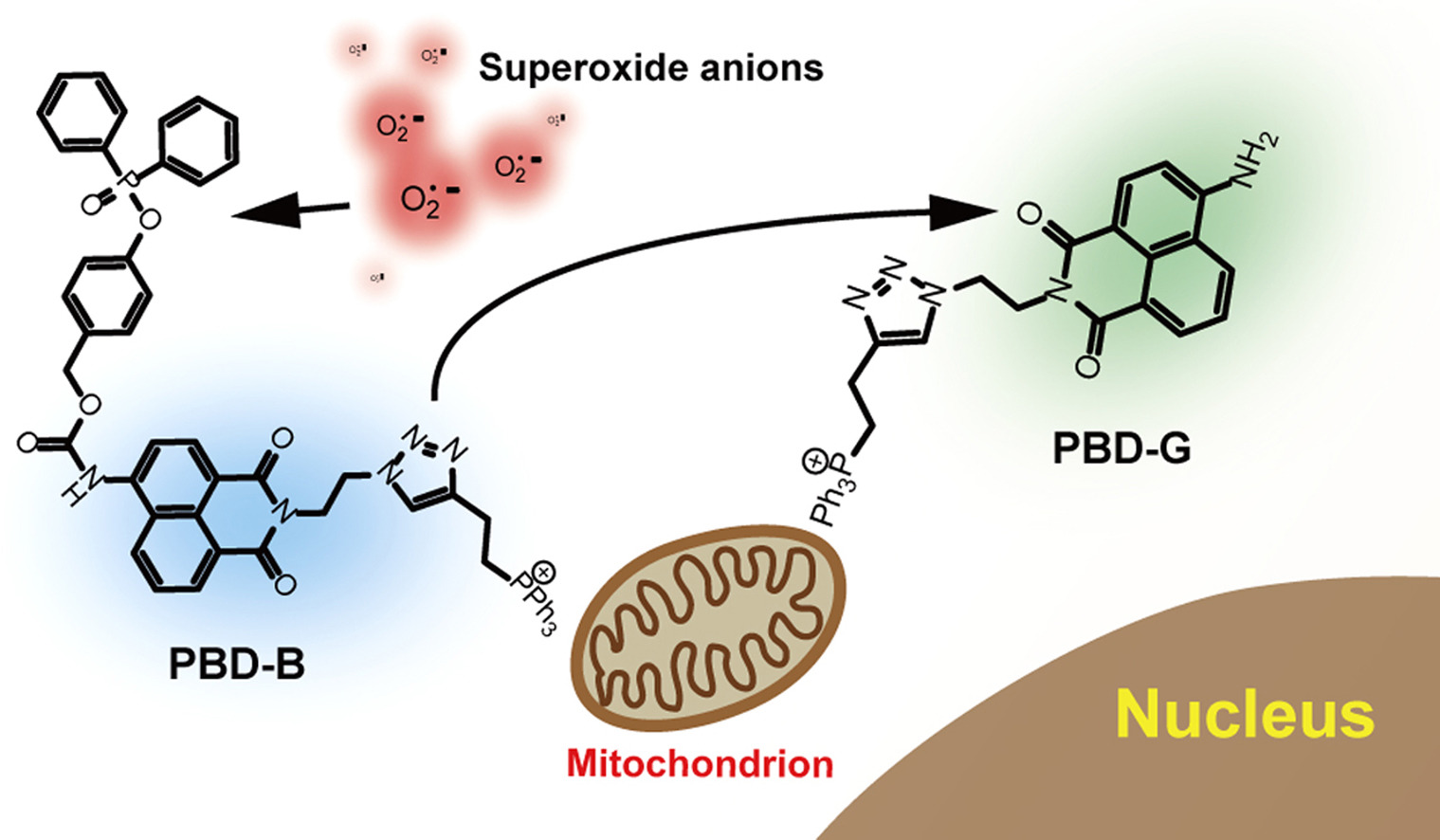
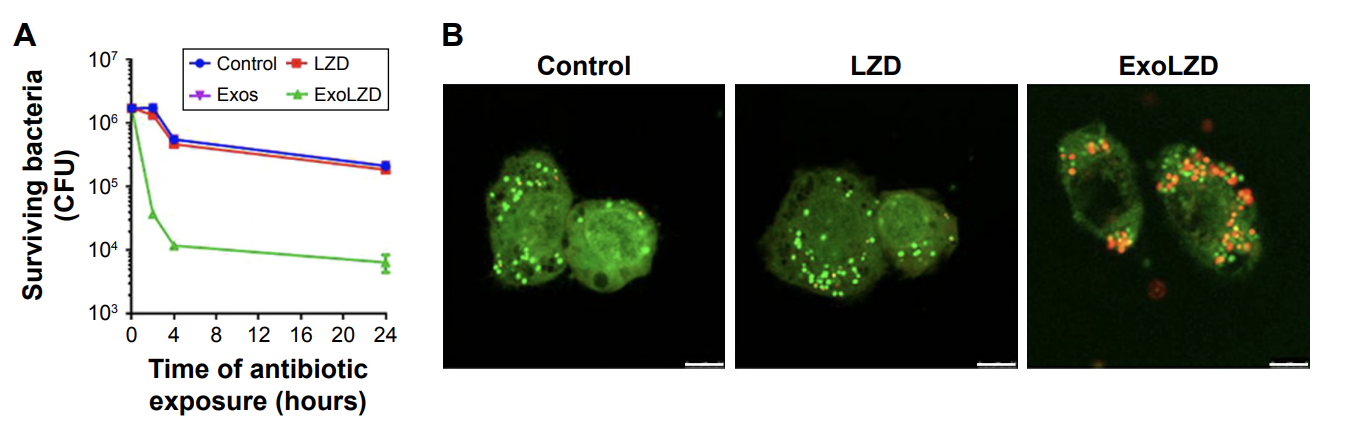
104. X. H. Yang, G. M. Shi, J. Guo, C. H. Wang, Y. He, Exosome-Encapsulated Antibiotic Against Intracellular Infections of Methicillin-Resistant Staphylococcus Aureus (MRSA), Int. J. Nanomed., 2018, 13, 8095—8104. DOI: 10.2147/IJN.S179380
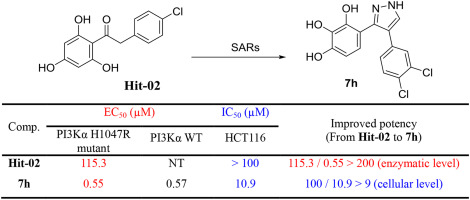
103. N. Zhang, Z. M. Yu, X. H. Yang, Y. Zhou,J. Wang, S. L. Zhang, M. W. Wang, and Y. He, Synthesis, Biological Evaluation and Structure-activity Relationship of a Novel Class of PI3Kα H1047R Mutant Inhibitors, Eur. J. Med. Chem., 2018, 158, 707-719. DOI: 10.1016/j.ejmech.2018.09.002
102. B. J. Xiong, X. Q. Zeng, S. S. Geng, S. Chen, B. Xu, Y. He, and Z. Feng, Thiyl Radical Promoted Chemo- and Regioselective Oxidation of C=C Bonds by Molecular Oxygen via Iron Catalysis, Green Chem., 2018, 20, 4521-4527. DOI: 10.1039/C8GC02369G

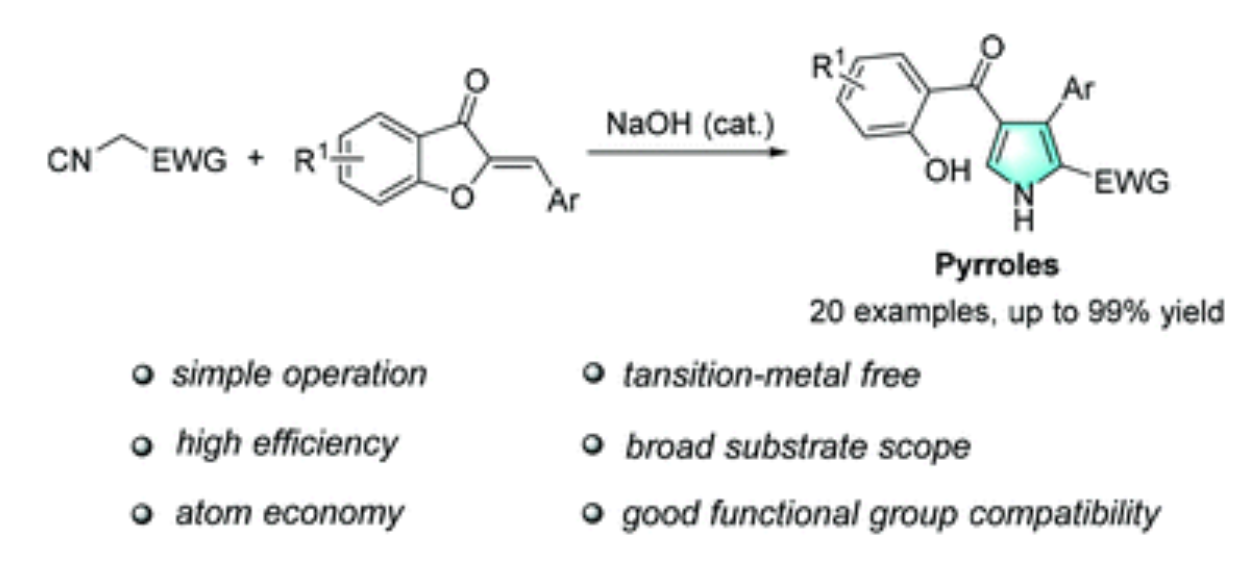
101. Z. P. Wang, Y. He and P. L. Shao, Transition-metal-free Synthesis of Polysubstituted Pyrrole Derivatives via Cyclization of Methyl Isocyanoacetate with Aurone Analogues, Org. Biomol. Chem., 2018, 16, 5422-5426. DOI: 10.1039/C8OB01558A
100. A. Yesu, Z. M. Yu, J. Ji, C. Zou, Z. Wang, and Y. He, Tandem Diels–Alder/Oxidation–Aromatization Reactions Involving 2-Styrylchromones and Arynes, Org. Biomol. Chem., 2018, 16, 6077-6085. DOI: 10.1039/C8OB01199K
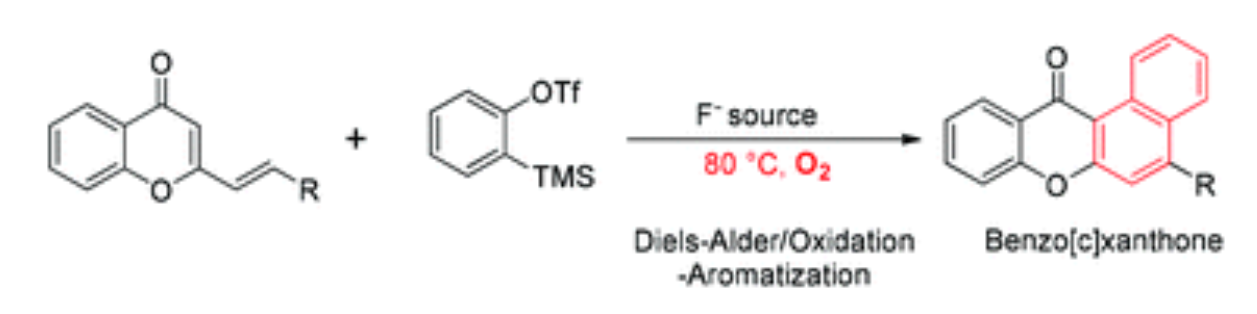
99. N. Zhang, Z. M. Yu, X. H. Yang, Y. Zhou, Q. Tang, P. Hu, J. Wang, S. L. Zhang, M. W. Wang, and Y. He, Difuran-substituted Quinoxalines as a Novel Class of PI3K<alpha> H1047R Mutant Inhibitors: Synthesis, Biological Evaluation and Structure-activity Relationship, Eur. J. Med. Chem., 2018, 157, 37-49. DOI: 10.1016/j.ejmech.2018.07.061

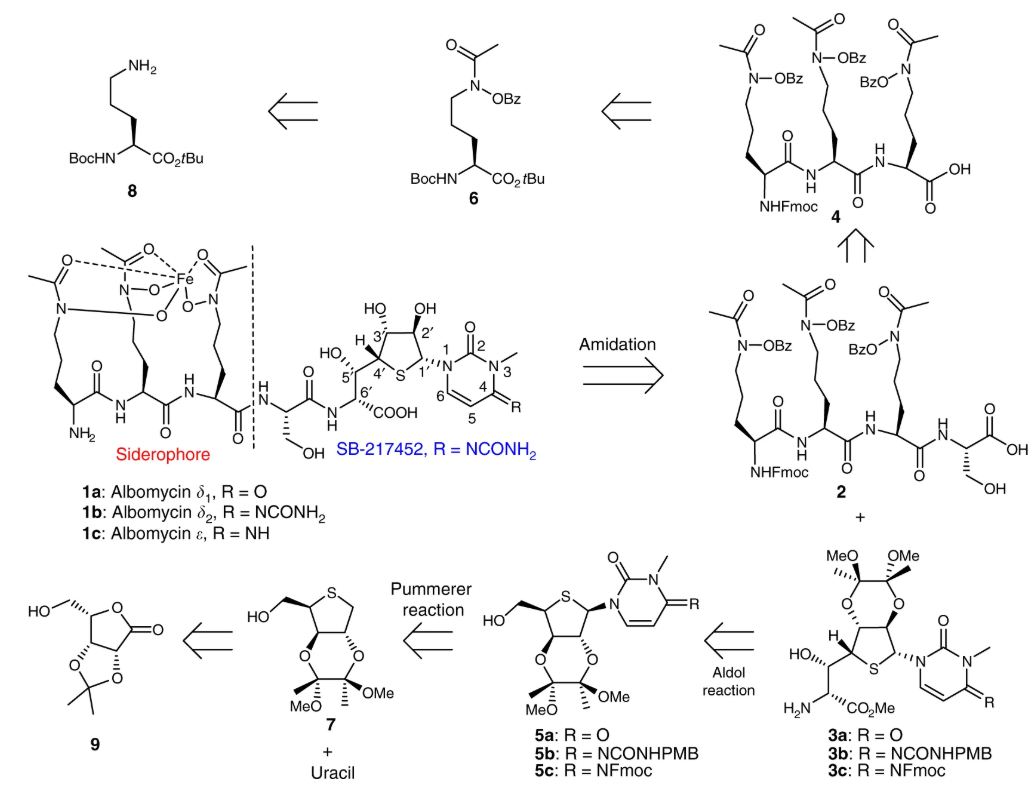
98. Z. H. Lin, X. B. Xu, S. Zhao, X. H. Yang, J. Guo, Q. Zhang, C. M. Jing, S. Chen and Y. He, Total Synthesis and Antimicrobial Evaluation of Natural Albomycins against Clinical Pathogens, Nat. Commun., 2018, 9, 3445. DOI: 10.1038/s41467-018-05821-1
New “Albomycin antibiotics discovered decades ago fall to total synthesis” https://cen.acs.org/pharmaceuticals/antibiotics/Albomycin-antibiotics-discovered-decades-agofall/96/web/2018/09
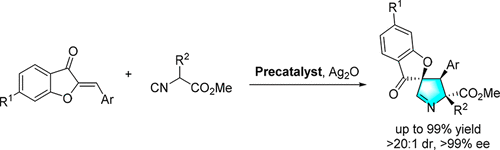
97. Z. P. Wang, S. C. Xiang, P. L. Shao, Y. He, Catalytic Asymmetric [3 + 2] Cycloaddition Reaction Between Aurones and Isocyanoacetates: Access to Spiropyrrolines via Silver Catalysis, J. Org. Chem., 2018, 83, 10995-11007. DOI: 10.1021/acs.joc.8b01622
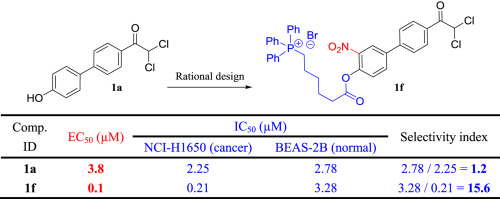
96. B. Xu, Z. M. Yu, S. C. Xiang, Y. S. Li, S. L. Zhang, Y. He, Rational Design of Mitochondria-targeted Pyruvate Dehydrogenase Kinase 1 Inhibitors with Improved Selectivity and Antiproliferative Activity, Eur. J. Med. Chem., 2018, 155 , 275-284. DOI:10.1016/j.ejmech.2018.06.012
95. S. Li, G. M. Li, X. H. Yang, Q. Meng, S. Yuan, Y. He, D. Q. Sun, Design, Synthesis and Biological Evaluation of Artemisinin Derivatives Containing Fluorine Atoms as Anticancer Agents, Bioorg. Med. Chem. Lett., 2018, 28, 2275-2278. DOI:10.1016/j.bmcl.2018.05.035
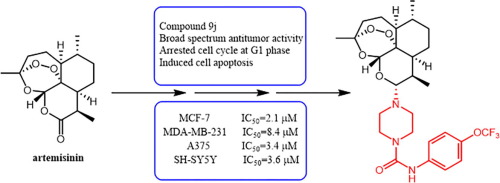
94. S. L. Zhang, Y. He, K. Y. Tam, Targeting Cancer Metabolism to Develop Human Lactate Dehydrogenase 5 (HLDH5) Inhibitors, Drug Discovery Today, 2018, 23, 1407-1415. DOI:10.1016/j.drudis.2018.05.014
93. M. H. Zhou, Q. Su, A. Yesu, Y. He and Z. Wang , Asymmetric Mannich Reaction of α-Diazocarbonyl Compounds and N-sulfonyl Cyclic Ketimines Catalyzed by Complexes Generated from Chiral and Achiral Phosphines with Gold(I), Org. Biomol. Chem., 2018, 16, 2923-2931. DOI: 10.1039/C8OB00577J
92. H. Y. You, S. Vegi, C. Lagishetti, S. Chen, R. S. Reddy, X. H. Yang, J. Guo, C. H. Wang, Y. He, Synthesis of Bioactive 3,4-Dihydro-2H-naphtho[2,3-b] [1,4]oxazine-5,10-dione and 2,3,4,5-Tetrahydro-1H-naphtho[2,3-b]azepine-6,11-dione Derivatives via Copper-catalysed Intramolecular Coupling Reaction, J. Org. Chem., 2018, 83, 4119-4130. DOI:10.1021/acs.joc.8b00020
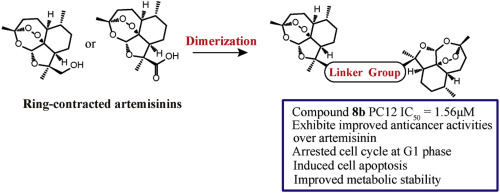
91. N. Zhang, Z. M. Yu, X. H. Yang, P. Hu, Y. He, Synthesis of Novel Ring-contracted Artemisinin Dimers with Potent Anticancer Activities, Eur. J. Med. Chem., 2018, 150, 829-840. DOI:10.1016/j.ejmech.2018.03.010
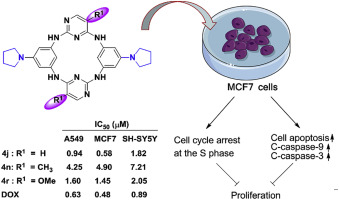
90. A. Yesu, X. H. Yang, M. H. Zhou, P. R. Devi, S. L. Zhang, Z. Wang, Y. He, Synthesis and Anticancer Activity Evaluation of Novel Azacalix[2]arene[2]pyrimidines, Eur. J. Med. Chem., 2018, 151, 214-225 DOI: 10.1016/j.ejmech.2018.02.079
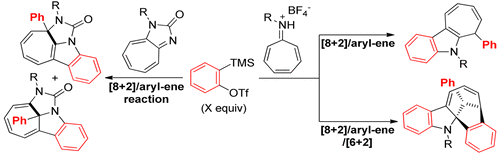
89. Z. Wang, A. Yesu, Y. He, Construction of Polycyclic Indole Derivatives via Multiple Aryne Reactions with Azaheptafulvenes, Org. Lett., 2018, 20, 644–647 DOI: 10.1021/acs.orglett.7b03789
88. Z. P. Wang; Q. Wu; J, Jiang; Z. R. Li; X. J. Peng; P. L. Shao; Y. He, Catalytic Asymmetric Synthesis of Pyrrolidine Derivatives Bearing Heteroatom-Substituted Quaternary Stereocenters, Org. Chem. Front., 2018, 5, 36-40 DOI:10.1039/C7QO00692F
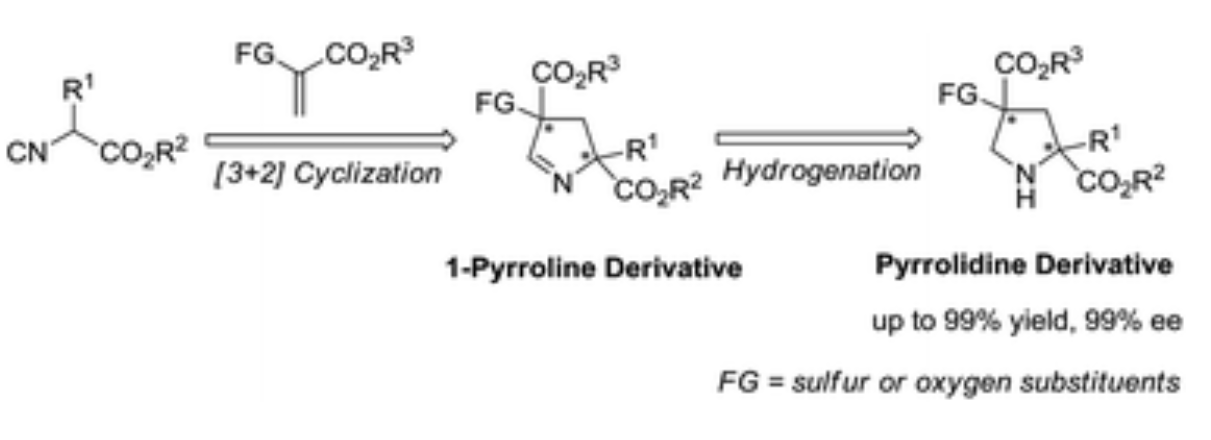
87. Z. P. Wang, Z. R. Li; Q. Wu, X. J. Peng, P. L. Shao, Y. He, Enantioselective Synthesis of 4-Thioetherpyrrolidine Derivatives via [3 + 2] Cycloaddition of α-Thioacrylates with Isocyanoacetates, J. Org. Chem., 2017, 82, 12869–12876 DOI:10.1021/acs.joc.7b02266
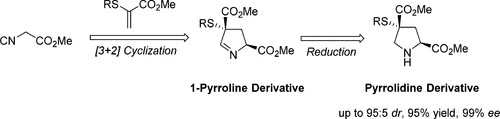
86. Palladium-catalyzed Oxidative Arylacetoxylation of Alkenes: Synthesis of Indole and Indoline Derivatives, Chem. Commun., 2017, 53, 11205-11208 DOI:10.1039/C7CC06448A
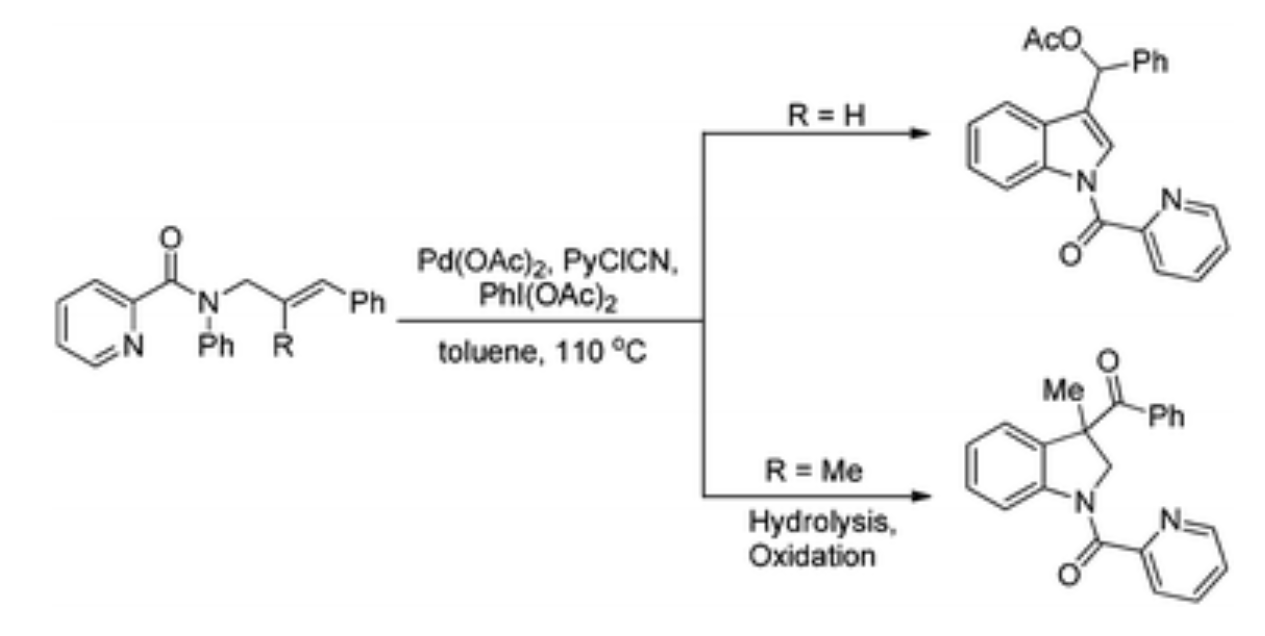
85. P. L. Shao, Z. R. Li, Z. P. Wang, M. H Zhou, Q. Wu, P. Hu, Y. He, [3 + 2] Cycloaddition of Azaoxyallyl Cations with Cyclic Ketones: Access to Spiro-4-oxazolidinones, J. Org. Chem., 2017, 82, 10680–10686 DOI: 10.1021/acs.joc.7b01728
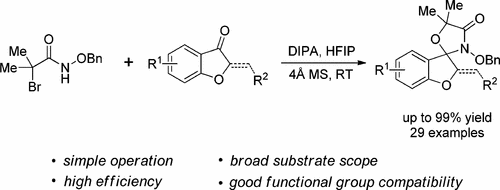
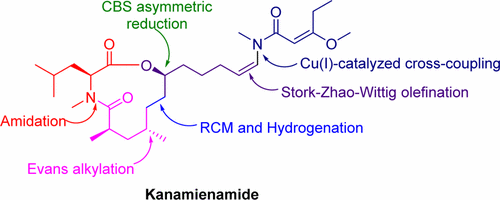
84.P. R. Devi; N. Zhang; Z. M. Yu; Z. Wang; Y. He, Total Synthesis of Kanamienamide, J. Org. Chem., 2017, 82, 11262–11268. DOI: 10.1021/acs.joc.7b01984
83. B. Sreenivas, P. R. Devi, W. X. Li, C. H. Wang, J. Guo, Y. He, A Unified Modular Synthetic Strategy for Dictyodendrins F, H, I, and G, Org. Lett., 2017,19, 4996–4999. DOI: 10.1021/acs.orglett.7b02511
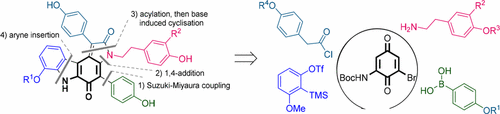
82. Q. Liu, P. Hu and Y. He, Asymmetric Total Synthesis of Nannocystin A, J. Org. Chem., 2017, 82, 9217–9222. DOI: 10.1021/acs.joc.7b01502
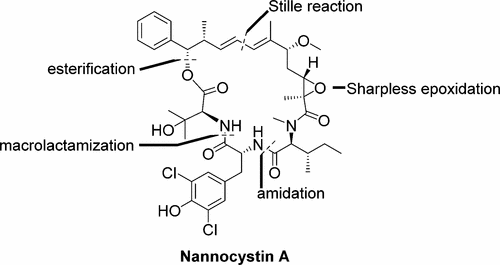
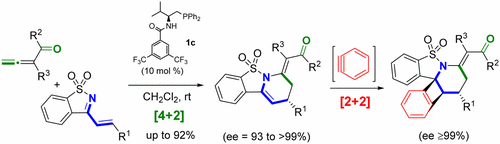
81. Z. Wang; H. C. Xu; Q. Su; P. Hu; P. L. Shao; Y. He; Y. X. Lu, Enantioselective Synthesis of Tetrahydropyridines/Piperidines via Stepwise [4 + 2]/[2 + 2] Cyclizations, Org. Lett., 2017,19, 3111-3114. DOI: 10.1021/acs.orglett.7b01221
80. M.Q. Tang; P. Hu; Q. Zheng; N. Tirelli; X. H. Yang; Z. L. Wang; Y. F. Wang; Q. Tang; Y. He, Polymeric Micelles with Dual Thermal and Reactive Oxygen Species (ROS)-Responsiveness for Inflammatory Cancer Cell Delivery, J. Nanobiotechnology, 2017, 15, 39. DOI: 10.1186/s12951-017-0275-4
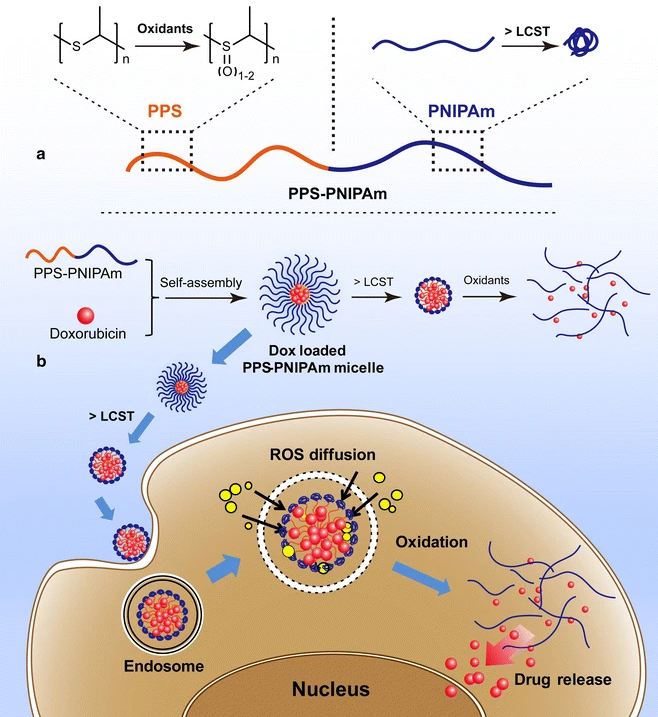
79. Q. Zheng, P. Hu, Q. Tang, M. Q. Tang, Z. L. Zang, P. L. Shao, Z. Wang, Y. He, Dually Responsive Amphiphilic Block Copolymer with Oxidation-Responsiveness and Tuneable LCST Behaviours, Mater. Lett., 2017, 201,133-136. DOI: 10.1016/j.matlet.2017.05.018
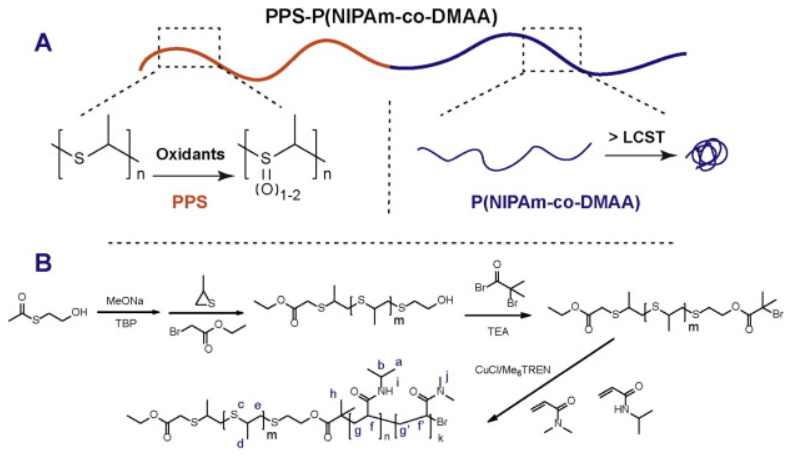
78. Z. L. Zang, S. Karnakanti, S. Zhao, P. Hu, Z. Wang, P.L. Shao, Y. He, Synthesis of Spiro-Dihydroquinoline and Octahydrophenanthrene Derivatives via Palladium-Catalyzed Intramolecular Oxidative Arylation, Org. Lett., 2017, 19, 1354-1357. DOI: 10.1021/acs.orglett.7b00228
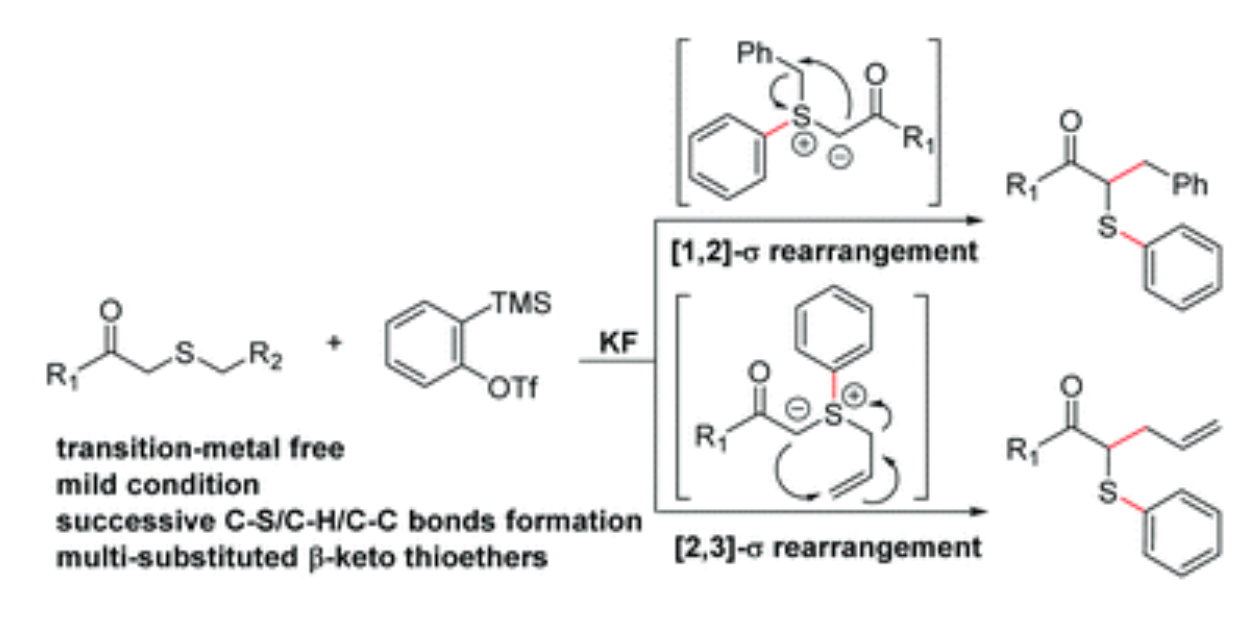
77. X. B. Xu, Z. H. Lin, Y. Y. Liu, J. Guo. Y. He, Stevens Rearrangement of Thioethers with Arynes: A Facile Access to Multi-Substituted β-Keto Thioethers, Org. Biomol. Chem., 2017,15, 2716-2720, DOI:10.1039/C7OB00277G
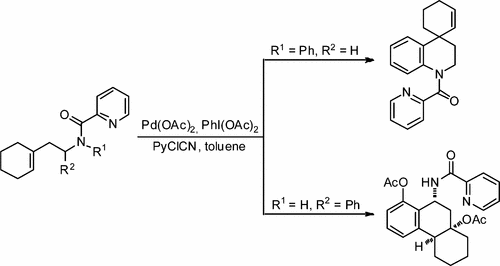
76. I. N. C Kiran, R. S. Reddy, C. Lagishetti, H. C. Xu, Z. Wang, Y. He, Selective Aza Diels–Alder and Domino [4+2]/[2+2] Cycloaddition Reactions of Arynes with N-Sulfonyl Ketimines, J. Org. Chem., 2017, 82, 1823-1832. DOI: 10.1021/acs.joc.6b02667
75. M. Q. Tang, Q. Zheng, N. Tirelli, P. Hu, Q. Tang, J. Gu, Y. He,Dual Thermo/Oxidation-Responsive Block Copolymers with Self-Assembly Behaviour and Synergistic Release, Reactive and Functional Polymers, 2017, 110, 55 –61. DOI: 10.1016/j.reactfunctpolym.2016.12.009

74. X. J. Peng, Y. A. Ho, Z. P. Wang, P. L. Shao, Y. Zhao, and Y. He, Formal [3+2] Cycloaddition of α‐Unsubstituted Isocyanoacetates and Methyleneindolinones: Enantioselective Synthesis of Spirooxindoles, Org. Chem. Front., 2017, 4, 81-85. DOI: 10.1039/C6QO00555A
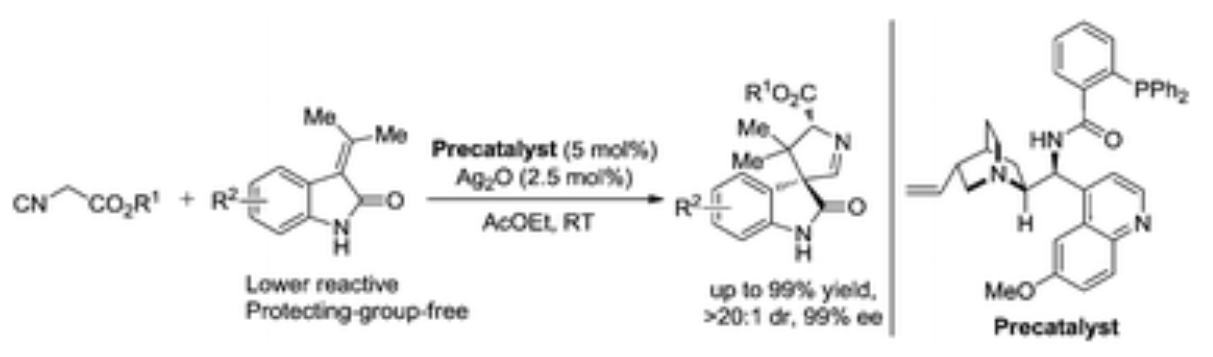
73. N. Qi, N. Zhang, A. S. Rao, J. S. Gao, J. Guo and Y. He, Insertion of Arynes into P–O Bonds: One-Step Simultaneous Construction of C–P and C–O Bonds, Org. Lett., 2016, 18, 6204–6207. DOI: 10.1021/acs.orglett.6b03283
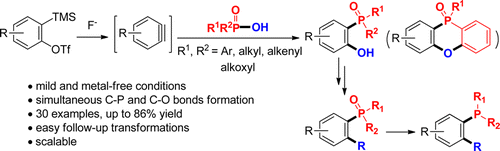
72. N. Qi, Z. L. Wang, A. S. Rao, Q. Liu, J. Guo, and Y. He, Total Syntheses of Anti-HIV Cyclodepsipeptides Aetheramides A and B, J. Org. Chem., 2016, 81, 12466–12471. DOI: 10.1021/acs.joc.6b02292
71. N. Qi, J. Guo, Y. He, Progress in Separation-Friendly Mitsunobu Reactions, Chin. J. Org. Chem., 2016, 36, 2858-2879. DOI: 10.6023/cjoc201606029

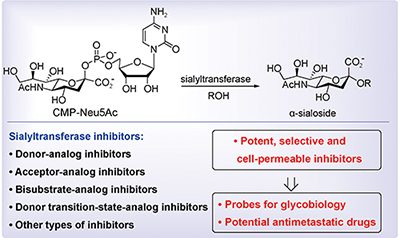
70. J. Guo, Y. He, X. S. Ye, Design and Discovery of Sialyltransferase Inhibitors. Progress in Chemistry., 2016, 12, 1712-1720. DOI: 10.7536/PC160442
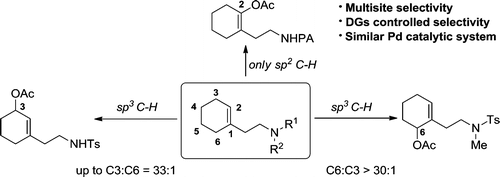
69. Z. L. Zang, S. Zhao, S. Karnakanti, C. L. Liu, P. L. Shao and Y. He, Catalytic Multisite-Selective Acetoxylation Reactions at sp2 vs sp3 C–H Bonds in Cyclic Olefins, Org. Lett., 2016, 18, 5014–5017. DOI: 10.1021/acs.orglett.6b02458
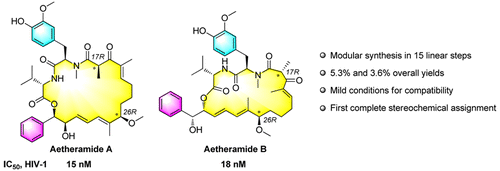
68. N. Qi, A. S. Rao, Z. L. Wang, Q. Liu, J. Guo, and Y. He, Asymmetric Total Syntheses of Aetheramides and Their Stereoisomers: Stereochemical Assignment of Aetheramides, Org. Lett., 2016, 18, 4718–4721. DOI: 10.1021/acs.orglett.6b02371
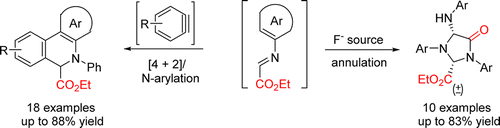
67. R. S. Reddy, C. Lagishetti, S. Chen, I. N. C. Kiran and Y. He, Synthesis of Dihydrophenanthridines and Oxoimidazolidines from Anilines and Ethylglyoxylate via Aza Diels–Alder Reaction of Arynes and KF-Induced Annulation, Org. Lett., 2016, 18, 4546–4549. DOI: 10.1021/acs.orglett.6b02186
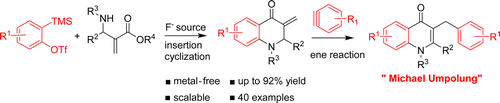
66. R. S Reddy, C. Lagishetti, I. N. C Kiran, H. Y. You, and Y. He, Transition-Metal-Free Cascade Synthesis of 4-Quinolones: Umpolung of Michael Acceptors via Ene Reaction with Arynes, Org. Lett., 2016, 18, 3818–3821. DOI: 10.1021/acs.orglett.6b01830
A Practical and Efficient Route to Heteraphanes: Synthesis of Structurally Simplified Analogues of Ansamycins, RSC Adv., 2016, 6, 68199-68203. DOI: 10.1039/C6RA16247A
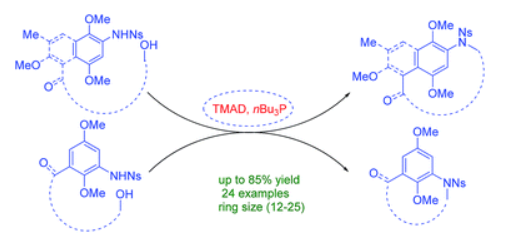
64. J. Guo, I.N. C. Kiran, J. S. Gao, R. S. Reddy, Y. He, Total Synthesis of Calothrixins and Their Analogues via Formal [3+2] Cycloaddition of Arynes and 2-Aminophenanthridinedione, Tetrahedron Lett., 2016, 57, 3481–3484. DOI: 10.1016/j.tetlet.2016.06.091

63. J. Guo, I. N. C Kiran, R. S Reddy, J. S. Gao, M. Q. Tang, Y. Y. Liu, and Y. He, Synthesis of Carbazolequinones by Formal [3 + 2] Cycloaddition of Arynes and 2-Aminoquinones, Org. Lett., 2016, 18, 2499–2502. DOI: 10.1021/acs.orglett.6b01090
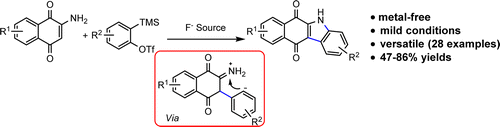
62. Y. He, P. K. Agarwal, I. N. C Kiran, R. C. Yu, B. Cao, C Zou, X. H. Zhou, H. C. Xu, B. Xu, L. Zhu, Y. Lan, K. C. Nicolaou, Efficient Synthesis of Dimeric Oxazoles, Piperidines and Tetrahydroisoquinolines from N-Substituted 2-Oxazolones, Chem. Eur. J., 2016, 22, 7696 –7701. DOI: 10.1002/chem.201601471
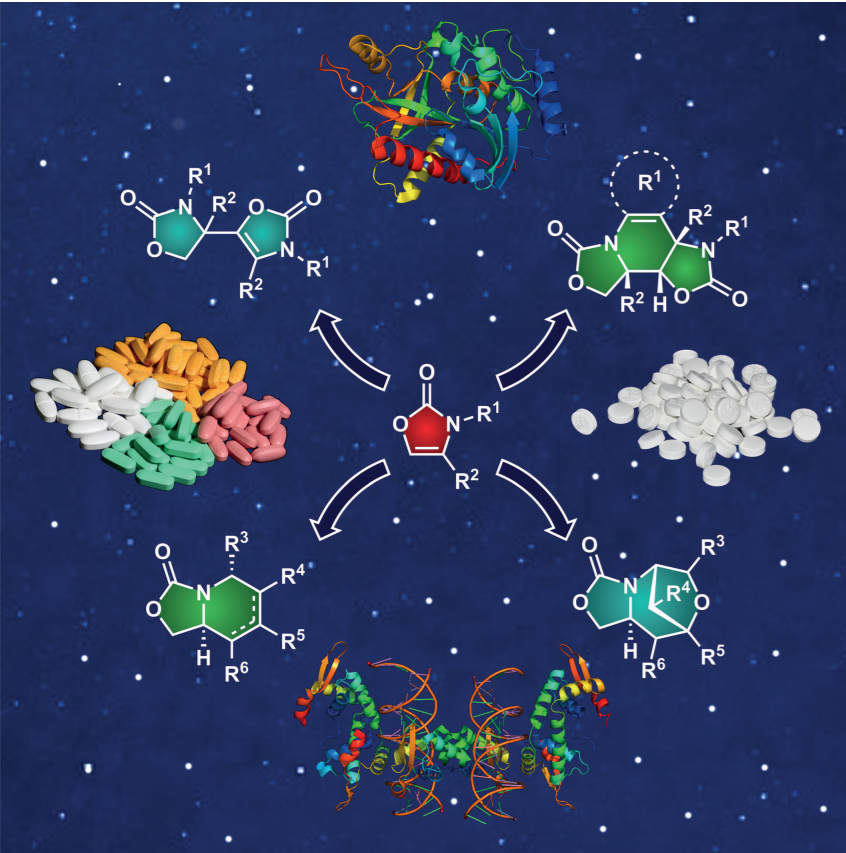
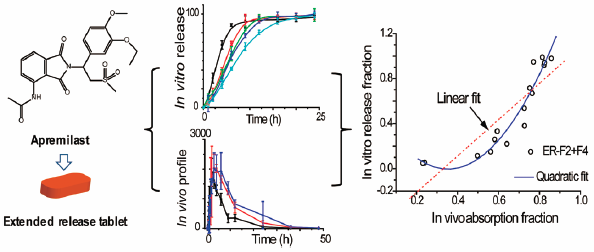
61. M. Q. Tang, P. Hu, S. G. Huang, Q. Zheng, H. Yu, Y. He, Development of an Extended-Release Formulation for Apremilast and a Level A in Vitro–in Vivo Correlation Study in Beagle Dogs, Chem. Pharm. Bull., 2016. 64, 1607–1615. DOI: http://doi.org/10.1248/cpb.c16-00519
60. J. C. Wong, G. Tang, X. Wu, C. Liang, Z. Zhang, L. Guo, Z. Peng, W. Zhang, X. Lin, Z. Wang, J. Mei, J. Chen, S. Pan, N. Zhang, Y. Liu, M. Zhou, L. Feng, Weili Zhao, S. Li, C. Zhang, M. Zhang, Y. Rong, T. Jin, X. Zhang, S. Ren, Ying Ji, R. Zhao, J. She, Y. Ren, C. Xu, D. Chen, J. Cai, S. Shan, D. Pan, Z. Ning, X. Lu, T. Chen, Y. He, L. Chen, Pharmacokinetic Optimization of Class-Selective Histone Deacetylase Inhibitors and Identification of Associated Candidate Predictive Biomarkers of Hepatocellular Carcinoma Tumor Response, J. Med. Chem., 2012, 55, 8903-8925. DOI: doi.org/10.1021/jm3011838

59. J. C. Wong, L. Guo, Z. Peng, W. Zhang, N. Zhang, W. Lai, Z. Zhang, C. Zhang, X. Zhang, S. Song, D. Pan, C. Xie, J. Li, Z. Ning, X. Lu, Y. He, L. Chen, Application of p21 and klf2 reporter gene assays to identify selective histone deacetylase inhibitors for cancer therapy, Bioorg. Med. Chem. Lett., 2011, 21, 110-116. DOI: 10.1016/j.bmcl.2010.11.063
58. G. Zhang, P. Ren, N.S. Gray, T. Sim, X. Wang, Y. Liu, J. Che, W. Dong, S.S. Tian, M.L. Sandberg, T.A. Spalding, R. Romeo, M. Iskandar, Z. Wang, M.H. Seidel, D.S. Karanewsky, Y. He, Discovery Of Pyrimidine Benzimidazoles as Src-family Selective Lck Inhibitors. Part II, Bioorg. Med. Chem. Lett., 2009, 19, 6691-6695. DOI:10.1016/j.bmcl.2009.09.123
57. S. Huang, Z. Liu, S.-S. Tian, M. Sandberg, T.A. Spalding, R. Romeo, M. Iskandar, Z. Wang, D.S. Karanewsky, Y. He, Discovery Of 2-Amino-6-Carboxamidobenzothiazoles As Potent Lck Inhibitors, Bioorg. Med. Chem. Lett., 2008, 18, 2324-2328. DOI: 10.1016/j.bmcl.2008.02.079
56. G. Zhang, P. Ren, N.S. Gray, T. Sim, Y. Liu, X. Wang, J. Che, S.-S. Tian, M.L. Sandberg, T.A. Spalding, R. Romeo, M. Iskandar, D. Chow H.M; Seidel, D.S. Karanewsky, Y. He, Discovery Of Pyrimidine Benzimidazoles As Lck Inhibitors: Part I, Bioorg. Med. Chem. Lett., 2008, 18, 5618-5621. DOI: 10.1016/j.bmcl.2008.08.104
55. T.H. Marsilje, P.B. Alper, W. Lu, D. Mutnick, P.-Y. Michellys, Y. He, D.S. Karanewsky, D. Chow, A. Gerken, J. Lao, M.-J. Kim, H.M Seidel, H. Martin; S.-S. Tian, Optimization Of Small Molecule Agonists Of The Thrombopoietin (TPO) Receptor Derived From A Benzo[A]Carbazole Hit Scaffold, Bioorg. Med. Chem. Lett., 2008, 18, 5259-5262. DOI: 10.1016/j.bmcl.2008.08.077
54. P. B. Alper, T.H. Marsilje, D. Mutnick, W. Lu, A. Chatterjee, M.J. Roberts, Y. He, D.S. Karanewsky, D. Chow, J. Lao, A. Gerken, T. Tuntland, B. Liu, J. Chang, P. Gordon, H. M. Seidel, S.-H. Tian, Discovery And Biological Evaluation Of Benzo[A]Carbazole-Based Small Molecule Agonists Of The Thrombopoietin (TPO) Receptor, Bioorg. Med. Chem. Lett., 2008, 18, 5255-5258. DOI: 10.1016/j.bmcl.2008.08.068
53. V. R. Gadhachanda, B. Wu, Z. Wang, K. L. Kuhen, J. Caldwell, H. Zondler, H. Walter, M. Havenhandb, Y. He, 4-Aminopyrimidines As Novel HIV-1 Inhibitors, Bioorg. Med. Chem. Lett., 2006, 16, 260-265. DOI: 10.1016/j.bmcl.2006.09.047
52. S. F. Yan, F. J. King, Y. He, J. S. Caldwell, Y. Zhou, Learning from the Data: Mining of Large High-Throughput Screening Databases, J. Chemical Information and Modeling, 2006, 46, 2381-2395.
51. D. Ellis, K. L. Kuhen, B. Anaclerio, B. Wu, K. Wolff, H. Yin, B. Bursulaya, J. Caldwell, D. Karanewsky, Y. He, Design, Synthesis And Biological Evaluations Of Novel Quinolones As HIV-1 Non-Nucleoside Reverse Transcriptase Inhibitors, Bioorg. Med. Chem. Lett., 2006, 16, 4246-4251. DOI:10.1016/j.bmcl.2006.01.053
50. Z. Wang, B. Wu, K. L. Kuhen, B. Bursulaya, T. N. Nguyen, D. G. Nguyen, Y. He, Synthesis And Biological Evaluations Of Sulfanyltriazoles As Novel HIV-1 Non-Nucleoside Reverse Transcriptase Inhibitors, Bioorg. Med. Chem. Lett., 2006, 16, 4174-4177.
49. Wu, K. Kuhen, T. N. Nguyen, D. Ellis, B. Anaclerio, X. He, K. Yang, D. Karanewsky, H. Yin, K. Wolff, K. Bieza, J. Caldwell, Y. He, Synthesis and Evaluation of N-Aryl Pyrrolidinones as Novel Anti-HIV-1 Agents. Part 1, Bioorg. Med. Chem. Lett., 2006, 16, 3430-3433. DOI:10.1016/j.bmcl.2006.04.012
48. H-S. Choi, Z. Wang, W. Richmond, X. He, K. Yang, T. Jiang, D. Karanewsky, X.-J. Gu, V. Zhou, Y. Liu, J. Che, C. Lee, J. Caldwell, T. Kanazawa, I. Umemura, N. Matsuura, O. Ohmori, T. Honda, N. Gray, Y. He, Design and Synthesis of 7H-Pyrrolo[2,3-d]pyrimidines as Focal Adhesion Kinase Inhibitors. II, Bioorg. Med. Chem. Lett., 2006, 16, 2689-2692
47. Jiang, K.L. Kuhen, K. Wolff, H. Yin, K. Bieza, J. Caldwell, B. Bursulaya, T.Y.-H. Wu, Y. He, Design, Synthesis and Biological Evaluations of Novel Oxindoles as HIV-1 Non-nucleoside Reverse Transcriptase Inhibitors. I, Bioorg. Med. Chem. Lett., 2006, 16, 2105-2108.
46. T. Jiang, K.L. Kuhen, K. Wolff, H. Yin, K. Bieza, J. Caldwell, B. Bursulaya, T. Tuntland, K. Zhang, D. Karanewsky, Y. He, Design, Synthesis and Biological Evaluations of Novel Oxindoles as HIV-1 Non-nucleoside Reverse Transcriptase Inhibitors. II, Bioorg. Med. Chem. Lett., 2006, 16, 2109-2112.
45. H-S. Choi, Z. Wang, W. Richmond, X. He, K. Yang, T. Jiang, T. Sim, D. Karanewsky, X.-J. Gu, V. Zhou, Y. Liu, O. Ohmori, J. Caldwell, N. Gray, Y. He, Design and Synthesis of 7H-Pyrrolo[2,3-d]pyrimidines as Focal Adhesion Kinase Inhibitors. I, Bioorg. Med. Chem. Lett., 2006, 16, 2173-2176.
44. H. Liu, D.C. Tully, R. Epple, B. Bursulaya, J. Li, J. L. Harris, J. A. Williams, R. Russo, C. Tumanut, M.J. Roberts, P.B. Alper, Y. He, D.S. Karanewsky, Design and Synthesis of Arylaminoethyl Amides as Noncovalent Inhibitors of Cathepsin S. Part 1, Bioorg. Med. Chem. Lett., 2005, 15, 4979-4984.
43. A. Kreusch, S. Han, A. Brinker, V. Zhou, H.-S. Choi, Y. He, S.A. Lesley, J. Caldwell, X.-J. Gu, Crystal Structures of Human HSP90α-Complexed with Dihydroxyphenylpyrazoles, Bioorg. Med. Chem. Lett., 2005, 15, 1475-1478.
42. V. Molteni, X. He, J. Nabakka, K. Yang, A. Kreusch, P. Gordon, B. Bursulaya, I. Warner, T. Shin, T. Biorac, N.S. Ryder, R. Goldberg, J. Doughty, Y. He, Identification of Novel Potent Bicyclic Peptide Deformylase Inhibitors, Bioorg. Med. Chem. Lett., 2004, 14, 1477-1481.
41. B. Wu, Y. He, “RNA Directed Small Molecule Drug Discovery.” Frontiers of Biotechnology & Pharmaceuticals 2004, 4, 314-337.
40. Y. He, J. Yang, B. Wu, L. Risen, R. Ranken, L. Blyn, K. Lowery, S. Hofstadler, E. E. Swayze, R. Griffey, “Synthesis and Biological Evaluations of Novel Benzimidazoles as Potential Antibacterial Agents.” Bioorg. Med. Chem. Lett. 2004, 14, 1217-1220. DOI:10.1016/j.bmcl.2003.11.031
39. Y. He, J, Yang, B. Wu, D. Robinson, K. Sprankle, P.-P. Kung, K. Lowery, V. Mohan, S. Hofstadler, E. E. Swayze, R. Griffey, “Synthesis and Evaluation of Novel Bacterial rRNA-binding Benzimidazoles by Mass Spectrometry.” Bioorg. Med. Chem. Lett. 2004, 14, 695-699.
38. Y. He, B. Wu, J. Yang, D. Robinson, L. Risen, R. Ranken, L. Blyn, R. Griffey, E. E. Swayze, “2-Piperidin-4-yl-benzimidazoles With Broad Spectrum Antibacterial Activities.” Bioorg. Med. Chem. Lett. 2003, 13, 3253-3256.
37. B. Wu, J. Yang, D. Robinson, S. Hofstadler, R. Griffey, E. E. Swayze, Y. He, “Synthesis of Linked Carbohydrates and Evaluation of Their Binding for 16S RNA by Mass Spectrometry.” Bioorg. Med. Chem. Lett. 2003, 13, 3915-3918.
36. B. Wu; J. Yang; Y. He; E. E. Swayze, “Reexamination of Neomycin B Degradation: Efficient Preparation of Its CD and D Rings as Protected Glycosyl Donors.” Org. Lett. 2002, 4, 3455-3458.
35. K.C. Nicolaou, J.-K. Jung, W. H. Yoon, K. C. Fong; H.-S. Choi; Y. He; Y.-L Zhong; P. S. Baran, “Total Synthesis of the CP-Molecules (CP-263,114 and CP-225,917, Phomoidrides B and A). 1. Racemic and Asymmetric Synthesis of Bicyclo[4.3.1] Key Building Blocks.” J. Am. Chem. Soc. 2002, 124, 2183-2189.
34. N-H. Lin; Y. Li; Y. He; M. W. Holladay; T. Kuntzweiler; D. J. Anderson; J. E. Campbell; S. P. Arneric, “Synthesis and structure-activity relationships of 5-substituted pyridine analogues of 3-[2-((S)-pyrrolidinyl)methoxy]pyridine, A-84543, a potent nicotinic receptor ligand.” Bioorg. Med. Chem. Lett. 2001, 11, 631-633
33. K.C. Nicolaou, J.-K. Jung, W.H. Yoon, Y. He, Y.-L. Zhong, P.S. Baran, “The Absolute Configuration and Asymmetric Total Synthesis of the CP Molecules (CP-263,114 and CP-225,917, Phomoidrides B and A).” Angew. Chem. Int. Ed. 2000, 39, 1829-1832.
32. K.C. Nicolaou, P.S. Baran, Y.-L. Zhong, H.-S. Choi, K.C. Fong, Y. He, and W.H. Yoon, “New Synthetic Technology for the Synthesis of Hindered a-Diazoketones via Acyl Mesylate.” Org. Lett. 1999, 1, 883-886.
31. K.C. Nicolaou, N.P. King, M.R.V. Finlay, Y. He, F. Roschangar, D. Vourloumis, H. Vallberg, F. Sarabia, S. Ninkovic, D. Hepworth, “Total Synthesis of Epothilone E and Related Side-Chain Modified Analogs via a Stille Coupling Based Strategy.” Bioorg. Med. Chem. 1999, 7, 665-697.
30. K.C. Nicolaou, P.S. Baran, Y.-L. Zhong, K.C. Fong, Y. He, W.H. Yoon and H.-S. Choi, “Total Synthesis of the CP Molecules CP-263,114 and CP-225,917 – Part 2: Evolution of the Final Strategy.” Angew. Chem. Int. Ed. 1999, 38, 1676-1678.
29. K.C. Nicolaou, P.S. Baran, Y.-L. Zhong, H.-S. Choi, W.H. Yoon, Y. He and K.C. Fong,” Total Synthesis of the CP Molecules CP-263,114 and CP-225,917 – Part 1: Synthesis of Key Intermediate and Intelligence Gathering.” Angew. Chem. Int. Ed. 1999, 38, 1669-1675.
C. Nicolaou, Y. He, K.C. Fong, W.H. Yoon, H.-S. Choi, Y.-L. Zhong, and P.S. Baran, “Novel Strategies to Construct the -Hydroxy Lactone Moiety of the CP Molecules. Synthesis of the CP-225,917 Core Skeleton.” Org. Lett. 1999, 1, 63-66.
C. Nicolaou, P.S. Baran, R. Jautelat, Y. He, K.C. Fong, H.S. Choi, W.Y. Yoon and Y.L. Zhong, “Novel Chemistry en Route to the CP Molecules: Unprecedented Construction of the Fused Malaic Anhydride Moiety.” Angew. Chem. Int. Ed. 1999, 38, 549-552.
C. Nicolaou, M.R.V. Finlay, S. Ninkovic, N.P. King, Y. He, T. Li, F. Sarabia, D. Vourloumis, “Synthesis and Biological Properties of C12,13-Cyclopropyl-Epothilone and Related Epothilones.” Chem. Biol. 1998, 5, 365-372.
25. K.C. Nicolaou, N.P. King, Y. He, “Ring-Closing Metathesis in the Synthesis of Epothilones and Polyether Natural Products.” in vol. titled Olefin Metathesis (Vol. Ed.: A. Fürstner) in Topics in Organometallic Chemistry, 1 (Alkene Metathesis in Organic Synthesis), 73-104. Springer-Verlag, Germany, 1998.
24. M. W. Holladay, H. Bai, Y. Li, N.-H. Lin, J.F. Daanen, K.B. Ryther, J.T. Wasicak, J.F. Kincaid, Y. He, A.-M. Hettinger, P. Huang, D.J. Anderson, A.W. Bannon, M.J. Buckley, J.E. Campbell, D.L. Donnelly-Roberts, K.L. Gunther, D.J.B. Kim, T.A. Kuntzweiler, J.P. Sullivan, M.W. Decker, S.P. Arneric, “Structure-Activity Studies Related to ABT-594, a Potent Nonopioid Analgesic Agent: Effect of Pyridine and Azetidine ring Substitutions on Nicotinic Acetylcholine Receptor Binding Affinity and Analgesic Activity in Mice.” Bioorg. Med. Chem. Lett. 1998, 8, 2797-2802.
23. R.C. Larock, Y. He, W.W. Leong, X. Han, M.D. Refvik, J.M. Zenner, “Palladium-Catalyzed Annulation of Allenes Using Functionally Substituted Vinylic Halides.” J. Org. Chem. 1998, 63, 2154-2160.
22. M.W. Holladay, J.T. Wasicak, N.-H. Lin, Y. He, K.B. Ryther, A.W. Bannon, M.J. Buckley, D.J.B. Kim, M.W. Decker, D.J. Anderson, J.E. Campbell, T.A. Kuntzweiler, D.L. Donnelly-Roberts, M. Piattoni-Kaplan, C.A. Briggs, M. Williams, S.P. Arneric, “Identification and Structure-Activity Relationships of (R)-5-(2-Azetidinylmethoxy)-2-chloropyridine (ABT-594), a Potent, Orally Active, Non-Opiate Analgesic Agent Acting via Neuronal Nicotinic Acetylcholine Receptors.” J. Med. Chem. 1998, 41, 407-412.
21. N-H. Lin, D.E. Gunn, Y. Li, Y. He, H. Bai, K.B. Ryther, T. Kuntzweiler, D.L. Donnelly-Roberts, D.J. Anderson, J.E. Campbell, J.P. Sullivan, S.P. Arneric, M.W. Holladay, “Synthesis and Structure-Activity Relationships of Pyridine-Modified Analogs of 3-[2-((S)-Pyrrolidinyl)methoxy]pyridine, A-84543, a Potent Nicotinic Acetylcholine Receptor Agonist.” Bioorg. Med. Chem. Lett. 1998, 8, 249-254.
20. K.C. Nicolaou, Y. He, F. Roschangar, N.P. King, D. Vourloumis, T. Li, “Total Synthesis of Epothilone E and Side-Chain Epothilone Analogs via the Stille Coupling Reaction.”Angew. Chem. Int. Ed. 1998, 37, 84-87. DOI: 10.1002/(SICI)1521-3773(19980202)37:1/2<84::AID-ANIE84>3.0.CO;2-V
19. K.C. Nicolaou, D. Vourloumis, T. Li, J. Pastor, N. Winssinger, Y. He, S. Ninkovic, F. Sarabia, H. Vallberg, F. Roschangar, N.P. King, M.R.V. Finlay, P. Giannakakou, P. Verdier-Pinard, E. Hamel, “Designed Epothilones: Solid Phase Synthesis on Microtubes, Tubulin Assembly Properties and Cytotoxic Action Against Taxol-Resistant Tumor Cells.” Angew. Chem. Int. Ed. 1997, 36, 2097-2103.
18. K.C. Nicolaou, F. Sarabia, M.R.V. Finlay, S. Ninkovic, N.P. King, D. Vourloumis, Y. He, “Total Synthesis of Oxazole- and Cyclopropane-Containing Epothilone B Analogs by the Macrolactonization Approach.” Chem. Eur. J. 1997, 3, 1971-1986.
17. K.C. Nicolaou, H. Vallberg, N.P. King, F. Roschangar, Y. He, D. Vourloumis, C.G. Nicolaou “Total Synthesis of Oxazole- and Cyclopropane-Containing Epothilone A Analogs by the Olefin Metathesis Approach.” Chem. Eur. J. 1997, 3, 1957-1970.
16. J.P. Sullivan, D. Donnelly-Roberts, C.A. Briggs, D.J. Anderson, M. Gopalakrishnan, I.C. Xue, M. Piattoni-Kaplan, E. Molinari, J.E. Campbell, D.G. Mckenna, D.E. Gunn, N.-H. Lin, K.B. Ryther, Y. He, M.W. Holladay, S. Wonnacott, M. Williams, S.P. Arneric, “ABT-089 [2-Methyl-3-(2-(S)-pyrrolidinylmethoxy)pyridine]: I. A Potent and Selective Cholinergic Channel Modulator with Neuroprotective Properties.” J. Pharmacol. Exp. Ther. 1997, 283, 235-246.
15. K.C. Nicolaou, S. Ninkovic, F. Sarabia, D. Vourloumis, Y. He, H. Vallberg, Z. Yang, “Total Synthesis of Epothilones A and B via a Macrolactonization Based Strategy.” J. Am. Chem. Soc. 1997, 119, 7974-7991.
14. K.C. Nicolaou*, Y. He, D. Vourloumis, H. Vallberg, F. Roschangar, F. Sarabia, S. Ninkovic, Z. Yang, “The Olefin Metathesis Approach to Epothilone A and Its Analogs.” J. Am. Chem. Soc. 1997, 119, 7960-7973. DOI: 10.1021/ja971109i
13. K.C. Nicolaou, N. Winssinger, J. Pastor, S. Ninkovic, F. Sarabia, Y. He, D. Vourloumis, Z. Yang, T. Li, P. Giannakakou, E. Hamel, “Synthesis of Epothilones A and B in Solid and Solution Phase.” Nature (London) 1997, 387, 268-272.
12. Yang, Y. He, D. Vourloumis, H. Vallberg, K.C. Nicolaou, “Total Synthesis of Epothilone A: The Olefin Metathesis Approach.” Angew. Chem. Int. Ed. 1997, 36, 166-168.
11. K.C. Nicolaou, Y. He, D. Vourloumis, H. Vallberg, Z. Yang, “An Approach to Epothilones Based on Olefin Metathesis.” Angew. Chem. Int. Ed. 1996, 35, 2399-2401.
10. Elliot, H. Kopecka, N.-H. Lin, Y. He, D. Garvey, “A Short, Efficient Synthesis of the Novel Cholinergic Channel Activator, ABT-418, from L-Proline.” Synthesis 1995, 772-774.
9. N-H. Lin, Y. He, S. P. Arneric, J. Sullivan, “Synthesis and Structure-Activity Relationships of 2’-(R)- and (S)-Pyrrolidine-Modified Analogs of the Cholinergic Channel Activator, ABT-418.” Bioorg. Med. Chem. Lett. 1995, 5, 1141-1146.
8. N-H. Lin, Y. He, H. Kopecka, “A Short, Efficient Chiral Synthesis of a Novel Cholinergic Channel Activator, ABT-418 [(S)-3-Methyl-5-(1-methyl-2-pyrrolidinyl)-isoxazole], from (S)-Pyroglutamic Acid.” Tetrahedron Lett. 1995, 36, 2563-2566.
7. D.S. Garvey, J. Wasicak, R. Elliott, S. Lebold, A-M, Hettinger, G. M. Carrera, N.-H., Lin, Y. He, M.W. Holladay, D.J. Anderson, E.D. Cadman, J. Raszkiewicz, J.P. Sullivan, S.P. Arneric, “Ligands for Brain Cholinergic Channel Receptors: Synthesis and in Vitro Characterization of Novel Isoxazoles and Isothiazoles as Bioisosteric Replacements for the Pyridine Ring in Nicotine.” J. Med. Chem. 1994, 37, 4455-4463.
6. N-H. Lin, Y. He, D. Anderson, J. Wasicak, R. Kasson, D. Sweeny, J. P. Sullivan and S. R. Arneric, “Synthesis and Structure-Activity Relationships of Pyrrolidine-Modified Analogs of Potent Cholinergic Channel Activator, ABT-418.” Bioorg. Med. Chem. Lett. 1994, 4, 2389-2392.
5. Y. He, N.-H. Lin, “Studies on Isoxazole Formation from Alkyl Carboxylic Esters.” Synthesis 1994, 989-992.
4. C.M. Lee, L.M. Jiang, H.S. Shang, P.M. Hon, Y. He, H.N.C. Wong, “Prehispanolone, a Novel Platelet Activating Factor Receptor Antagonist from Leonurus Heterophylius.” Br. J. Pharmacol. 1991, 103, 1719-1724.
3. Y. He, H.M. Chang, Y.K. Lau, Y.X. Cui, R.J. Wang, T.C.W. Mak, H.N.C. Wong, C.M. Lee, “Synthesis of Diazepam Related Analogues of Miltirone, an Active Central Benzodiazepam Receptor Ligand from Salvia Miltiorrhiza Bunge (Danshen).” J. Chem. Soc. Perkin Transactions I 1990, 3359-3361.
2. Y. He, H. Zhao, X.F. Pan, S.F. Wang, “Reduction with Metal Borohydride-Transition Metal Salt System II.” Synth. Commun. 1989, 19, 3051-3054.
1. Y. He, H. Zhao, X.F. Pan, S.F. Wang, “Reduction with Metal Borohydride-Transition Metal Salt System I.” Synth. Commun. 1989, 19, 3047-3050.
Patents
76. 邹成, 周兴华, 曹蓓, 徐化成, P•K•阿加瓦尔, C•K•I•那迦, 贺耘. “N-取代恶唑酮类聚合衍生物的制备方法” 中国. CN104788396A. 2015.07.22.
75. 段方方, V•S•拉奥, 郑绍军, 游恒耀, C•拉吉施提;R•S•雷迪, 贺耘. “制备红迪菌素的三环分子骨架的方法” 中国. CN104817514A. 2015.08.05.
74. 周兴华, 王志鹏, 彭小娇, 郑绍军, 邵攀霖, 贺耘. “三嗪衍生物的不对称合成方法” 中国. CN104817560A. 2015.08.05.
73. 曹蓓, 邹成, 周兴华, 徐化成, 徐标, P·K·阿加瓦尔, C·K·I·那迦, 贺耘. “恶唑酮类杂环化合物的制备方法” 中国. CN106045935A. 2016.10.26.
72. Chen, Li; Feng, Lichun; Y, He; Huang, Mengwei; Liu, Yongfu; Yun, Hongying; Zhou, Mingwei “Preparation of novel tetrahydroquinoline derivatives as AMPK activators” WO 2012001020.
71. L. Chen, M. Huang, L. Feng, Y. He, H. Yun, “Spiro-cyclopropane-indolinone derivatives as AMPK modulators and their preparation, pharmaceutical compositions and use in the treatment of diseases” WO2011069298
70. L. Chen, L. Feng, M. Huang, Y. He, H. Yun, “Novel cyclopropane-indolinone derivatives” US 20110144106
69. J. B. Blanc, L. Chen, F. Firooznia, P. Gillespie, R.A. Goodnow, Y. He, T.A. Lin, S.S. So, H. Yun, Z. Zhang, “Preparation of bi-aryl aminotetralines as CRTH2 receptor antagonists” WO 2010018113
68. J. B. Blanc, L. Chen, F. Firooznia, P. Gillespie, R.A. Goodnow, Y. He, T.A. Lin, S.S. So, H. Yun, Z. Zhang, “Bi-aryl aminotetralines” US 20100041714
67. L. Chen, F. Firooznia, P. Gillespie, Y. He, T.A. Lin, S.S. So, H. Yun, “Aminotetrahydroindazoloacetic acids” US 20100016368
66. L. Chen, F. Firooznia, P. Gillespie, Y. He, T.A. Lin, E. Mertz, S.S. So, H. Yun, Z. Zhang, “Preparation of naphthylacetic acids as antagonists or partial agonists at the CRTH2 receptor.” WO 2010055004
65. L. Chen, F. Firooznia, P. Gillespie, Y. He, T.A. Lin, S.S. So, H. Yun, Z. Zhang, “Preparation of 4,5,6,7-tetrahydroindazolyl acetic acids as antagonists of CRTH2 receptor” WO 2010006944
64. L. Chen, F. Firooznia, P. Gillespie, Y. He, T.A. Lin, S.S. So, H. Yun, Z. Zhang, “Aminotetrahydroindazoloacetic acids” US 8138208 (2009)
63. L. Chen, F. Firooznia, P. Gillespie, Y. He, T.A. Lin, S.S. So, H. Yun, “Aminotetrahydroindazoloacetic acids” US 8124641 (2009)
62. L. Chen, F. Firooznia, P. Gillespie, Y. He, T.A. Lin, E. Mertz, S.S. So, H. Yun, Z. Zhang, “Naphthylacetic acids” US 8124629 (2012)
61. F. Firooznia, P. Gillespie, Y. He, T.A. Lin, S.S. So, H. Yun, “Preparation of 4,5,6,7-tetrahydroindazolyl acetic acids as antagonists of CRTH2 receptor” WO 2010006939
60. L. Chen, Y. He, J.C. Wong, “Preparation of novel N-(2-aminophenyl) amide derivatives as antitumor agents” WO 2009095324
59. L. Chen, Y. He, J.C. Wong, “N-(2-amino-phenyl)-amide derivatives” US 7795315 (2009)
58. L. Chen, Y. He, J.C. Wong, “Novel N-(2-amino-phenyl)-amide derivatives” US 20090203681
57. L. Chen, X. Han, Y. He, S. Yang, Z. Zhang, “Spiroindolinone derivatives as interaction inhibitors between p53 and MDM2 proteins and their preparation, pharmaceutical compositions and use in the treatment of cancer” US 2009163512
56. T. Sim, T.N. Nguyen, B. Wu, Y. He, Y. Xie, X. Wang, G. Zhang, N.S. Gray, “Preparation of quinazolinecarboxamides as protein kinase inhibitors” US 20110053932
55. L. Chen, X. Han, Y. He, S. Yang, Z. Zhang, “Spiroindolinone derivatives” US 7776875 (2008)
54. P. Ren, X. Wang, S. Jiang, A. Nagle, B. Okram, Y. He, Y. Xie, X. Wang, S. Huang, X. Wang, Y. Liu, P.A. Albaugh, N.S. Gray, Nathanael G. Zhang, “Preparation of pyrrolo[2,3-b]pyridinecarboxamides as protein kinase inhibitors” WO 2008144253
53. Y. He, Z. Liu, S. Huang, “Preparation of benzothiazole derivatives as protein kinase inhibitors” WO 2008124393
52.P. Ren, X. Wang, Y. He, B. Okram, X. Wang, G. Zhang, Z. Liu, Y. Liu, P.A. Albaugh, “Preparation of substituted pyrazolopyrimidinamines as protein kinase inhibitors” WO 2008112695
51. P. A. Albaugh, Y. He, S. Jiang, P. Ren, X. Wang, X. Wang, Y. Xie, “[4,5′]bipyrimidinyl-6,4′-diamine derivatives as protein kinase inhibitors” US 8026243 (2011)
50. Y. He, Z. Wang, B. Wu, “Terephthalamate compounds and compositions, and their use as HIV integrase inhibitors” US 20100016379
49. D. Chianelli, C. Cow, Y. He, S. Jiang, X. Li, X. Liu, Z. Liu, J. Loren, V. Molteni, J. Nabakka, P. Ren, T. Sim, X. Wang, S. You, “Compositions and methods for modulating c-kit and PDGFR receptors” US 7678792B2 (2010)
48. D. Chianelli, C. Cow, Y. He, S. Jiang, X. Li, X. Liu, Z. Liu, J. Loren, V. Molteni, J. Nabakka, P. Ren, T. Sim, X. Wang, S. You, “Compositions and methods for modulating c-kit and PDGFR receptors” US 20100081656
47. P. A. Albaugh, Y. He, S. Jiang, P. Ren, X. Wang, X. Wang, Y. Xie, “[4,5′]bipyrimidinyl-6,4′-diamine derivatives as protein kinase inhibitors” US 20100234376
46. P. Ren, G. Zhang, S. You, T. Sim, N. Gray, Y. Xie, X. Wang, Xing, Y. He, “Compounds and methods for FGF Receptor kinases inhibitors” US 20090312321
45. T. Marsilje, W. Lu, P. Alper, D. Mutnick, Y. He, “Preparation of indolyl or benzimidazolyl benzoic acids as TPO mimetics” US 7816542B2 (2010)
44. E.E. Swayze, Y. He, P. P. Seth, E. A. Jefferson, “Benzimidazole compounds” US 7244847 (2007)
43. P. Alper, T. Marsilje, A. Chatterjee, W. Lu, D. Mutnick, M. J. Roberts, Y. He, “Preparation of benzo[a]carbazole and indeno[1,2-b]indole derivatives as TPO mimetics” US 20090075996
42. B. Wu, T. Nguyen, D.A. Ellis, X. He, B. Anaclerio, K. Yang, H.-S. Choi, Z. Wang, T. Marsilje, Y. He, “Phenyl-substituted pyrrolidones” US 20080287516
41. Y. He, D. Woodmansee, H.-S. Choi, Z. Wang, B. Wu, T. Nguyen, “Synthesis of aryl pyrrolidones” WO 2006081562
40. HLiu, X. He, H.-S. Choi, K. Yang, D. Woodmansee, Z. Wang, D.A. Ellis, B. Wu. Y. He, T. N. Nguyen, “Preparation of pyrazolopyrimidinones and analogs, and their compositions as cannabinoid CB1 receptor inhibitors” US 20090247517
39. S, Pan, T.H. Marsilje, W. Lu, W. Gao, N.S. Gray, He, Y. Liu, Y. Mi, Y. Xie, “Immunosuppressant compounds and compositions” US 20070203100
38. H-S. Choi, Z. Wang, N.S. Gray, X.-J. Gu, X. He, Y. He, T. Jiang, Tao, Y. Liu, W. Richmond, T. Sim, K. Yang, “Compounds and compositions as protein kinase inhibitors” US 20070225306
37. H-S. Choi, Z. Wang, N.S. Gray, X.-J. Gu, X. He, Y. He, T. Jiang, Tao, Y. Liu, W. Richmond, T. Sim, K. Yang, “Substituted pyrrolo[2,3-2]pyrimidines as protein kinase inhibitors”US 7968557 (2010)
36. H. Liu, A. Chatterjee, A.; D.C. Tully, P.B. Alper, B. Bursulaya, J. Guo, D. Woodmansee, D. Mutnick, D.S. Karanewsky, Y. He, “Inhibitors of cathepsin S and pharmaceutical compositions containing cathepsin S inhibitors” US 7501408 (2009)
35. S, Pan, T.H. Marsilje, W. Lu, W. Gao, N.S. Gray, He, Y. Liu, Y. Mi, Y. Xie, “Immunosuppressant compounds and compositions” US 7572811B2 (2009)
34. H. Liu, A. Chatterjee, A.; D.C. Tully, P.B. Alper, B. Bursulaya, J. Guo, D. Woodmansee, D. Mutnick, D.S. Karanewsky, Y. He, “Inhibitors of cathepsin S and pharmaceutical compositions containing cathepsin S inhibitors” US 7314872B2 (2008)
33. V. Molteni, X. He, Y. He, A. Kreusch, J. Nabakka, K. Yang, “Bicyclic compounds and compositions as PDF inhibitors” US 7579363 (2009)
32. Y. He, E.E. Swayze, P.P. Seth, E.A. Jefferson, “Preparation of piperidinylbenzimidazoles and analogs thereof as antibacterials” WO 2004047769
31. Y. He, D.A. Ellis, B. M. Anaclerio, K.L. Kuhen, B. Wu, T. Jiang, “Quinolones with anti-HIV activity, and preparation thereof” US 7019141B2 (2006)
30. B. Wu, Y. He, T. Ngyuen, K.L. Kuhen, D.A. Ellis, T. Jiang, X. He, K. Yang, B. Bursulaya, “Pyrrolidones with anti-HIV activity” US 7495016B2 (2009)
29. B. Wu, Y. He, T. Ngyuen, K.L. Kuhen, D.A. Ellis, T. Jiang, X. He, K. Yang, B. Bursulaya, “Pyrrolidones with anti-HIV activity” US 7601742 (2009)
28. Y. He, T. Jiang, K.L. Kuhen, D.A. Ellis, B. Wu, T. Y.-H. Wu, B. Bursulaya, “Oxindoles for treatment of HIV infection, and preparation thereof” WO 2004037247
27. Y. He, Tao Jiang, Kelli L. Kuhen, David Archer Ellis, Baogen Wu, Tom Yao-Hsiang Wu, Badry Bursulaya “Oxindoles with anti-HIV activity” US 7205328 B2 (2007)
26. Y. He, Tao Jiang, Kelli L. Kuhen, David Archer Ellis, Baogen Wu, Tom Yao-Hsiang Wu, Badry Bursulaya “Oxindoles with anti-HIV activity” US 20040152755
25. E. E. Swayze, Y. He, P.P. Seth, E.A. Jefferson, “Preparation of novel benzimidazole compounds as antibacterial agents” WO 2003066622
24. K.C. Nicolaou, Y. He, S. Ninkovic, J. Pastor, F. Roschangar, F. Sarabia, H. Vallberg, D. Vourloumis, N. Winssinger, Z. Yang, N.P. King, M.R. Finlay, “Epothilone analogs” US 6441186B1 (2002)
23. E.E. Swayze, Y. He, P.P. Seth, E.A. Jefferson, “Benzimidazole compounds” US 7244847B2 (2007)
22. N.H. Lin, Y. He, M.W. Holladay, K.B. Ryther, Y.L. Li, H. Bai, “3-Pyridyloxymethyl heterocyclic ether compounds with nicotinic cholinergic activity, useful in controlling chemical synaptic transmission” US 6127386 (2000)
21. K.C. Nicolaou, Y. He, S. Ninkovic, J. Pastor, F. Roschangar, F. Sarabia, H. Vallberg, D. Vourloumis, N. Winssinger, Z. Yang, N.P. King, M.R. Finlay, “Epothilone analogs” US 6660758 (2003)
20. K.C. Nicolaou, N.P. King, M.R. Finlay, Y. He, F. Roschangar, D. Vourloumis, H. Vallberg,F. Sarabia, S. Ninkovic, D. Hepworth, T. Li, “Preparation of epothilone analogs possessing microtubule stabilizing effects and cytotoxicity” US 6380394 (2002)
19. V. Molteni, X. He, Y. He, A. Kreusch, J. Nabakka, K. Yang, “Bicyclic compounds and compositions as PDF inhibitors” US 7253164 (2007)
18. M.W. Holladay, M.A. Abreo, D.E. Gunn, N.-H. Lin, D.S. Garvey, K.B. Ryther, S.A. Lebold, R.L. Elliott, Y. He, J.T. Wasicak, H. Bai, M.J. Dart, P.P. Ehrlich, Y.L. Li, J.F. Kincaid, J.M. Schkeryantz, J.K. Lynch, “Heterocyclic Ether and Thioether Compounds Useful in Controlling Chemical Synaptic Transmission” WO 9932480
17. K.C. Nicolaou, N.P. King, M.R.V. Finlay, Y. He, F. Roschangar, D. Vourloumis, H. Vallberg, A. Bigot, “Epothilone Derivatives and their synthesis and use” US 20030203938
16. K.C. Nicolaou, Y. He, S. Ninkovic, J. Pastor, F. Roschangar, F. Sarabia, H. Vallberg, D. Vourloumis, N. Winssinger, Z. Yang, N.P. King, M.R. Finlay, M. R. Verschoyle, “Epothilone analogs” US 7173137 (2003)
15. K.C. Nicolaou, N.P. King, M.R.V. Finlay, Y. He, F. Roschangar, D. Vourloumis, H. Vallberg, A. Bigot, “Epothilone Derivatives and their synthesis and use” US 6531497 (2003)
14. K.C. Nicolaou, N.P. King, M.R.V. Finlay, Y. He, F. Roschangar, D. Vourloumis, H. Vallberg, A. Bigot, “Epothilone Derivatives and their synthesis and use” US 7579366 (2009)
13. N.H. Lin, M.W. Holladay, M.A. Abreo, D.E. Gunn, S.A. Lebold, R.L Elliott, Y. He, “Heterocyclic Ether Compounds Useful in Controlling Neurotransmitter Release” US 5914328 (1999)
12. R.L. Elliott, K.B. Ryther, M.W. Holladay, J.T. Wasicak, J.F. Daanen, N.-H. Lin, M.J. Dart, Y. He, Y. Li, “Furopyridine, Thienopyridine, Pyrrolopyridine Useful in Controlling Chemical Synaptic Transmission” US 6001849 (1999)
11. K.C. Nicolaou, Y. He, S. Ninkovic, J. Pastor, F. Roschangar, F. Sarabia, H. Vallberg, D. Vourloumis, N. Winssinger, Z. Yang, N.P. King, M.R.V. Finlay, “Epothilone Analogs” US 20040127432
10. N-H. Lin, Y. He, S.J. Wittenberger, “Method of Preparing Enantiomerically-Pure 3-Methyl-5-[1-alkyl-2-(S)-pyrrolidinyl]isoxazoles for Pharmaceutical use” US 5516912 (1996)
9. N-H. Lin, Y. He, S.J. Wittenberger, “Method of Preparing Enantiomerically-Pure 3-Methyl-5-[1-alkyl-2-(S)-pyrrolidinyl]isoxazoles” US 5559242 (1996)
8. M. W. Holladay, N.-H. Lin, K.B. Ryther, Y. He, “Preparation of 2-(3-Pyridyloxyalkyl)azetidines and -Pyrrolidines as Nicotinic Receptor Ligands” WO 9640682
7. N-H. Lin, Y. He, M.W. Holladay, K.B. Ryther, Y. Li, “3-Pyridyloxymethyl Heterocyclic Ether Compounds Useful as Nicotinic Cholinergic Ligands in Controlling Chemical Synaptic Transmission” US 5629325 (1997)
6. N-H. Lin, Y. He, M.W. Holladay, K.B. Ryther, H. Bai, “3-pyridyloxymethyl heterocyclic ether compounds useful in controlling chemical synaptic transmission” US 6437138 (2002)
5. N-H. Lin, Y. He, M.W. Holladay, K.B. Ryther, H. Bai, “3-pyridyloxymethyl heterocyclic ether compounds useful in controlling chemical synaptic transmission” US 6127386 (2000)
4. D. Garvey, G.M. Carrera, S. Arneric, Y.K. Shue, N.-H. Lin, Y. He, “Isoxazole and Isothiazole Compounds That Enhance Cognitive Function” WO 922133
3. N-H. Lin, Y. He, W.H. Bunnelle, “Process for the Preparation of Enantiomerically Pure 3-Methyl-5-[1-(C1-C3-Alkyl)-2-pyrrolidinyl]isoxazole” US 5508418 (1996)
2. N-H Lin, Y. He, R.L. Elliot, M.S. Chorghade, T.K. J. Esch, D.O. Beer, C.C. Witzig, C. Christian, T.C. Herzig, S.J. Wittenberger, W. H. Bunnelle, B. A. Narayanan, P. R. Singam, A. V. Rama Rao, “Method of preparing enantiomerically-pure 3-methyl-5-(1-alkyl-2(S)-pyrrolidinyl) isoxazoles” US 5424444 (1995)
1. D. Garvey, G.M. Carrera, S. Arneric, Y.K. Shue, N.-H. Lin, Y. He, E. L. Lee, S. A. Lebold, “Isoxazole, isothiazole and pyrazole compounds that Enhance cognitive function” US 5409946 (1995)